Abstract
The intracellular life of Listeria monocytogenes starts by a complex process of entry involving several bacterial ligands and eukaryotic receptors. In this work, we identified in silico from the sequence of the genome of L. monocytogenes a previously unknown gene designated lpeA (for lipoprotein promoting entry) encoding a 35-kDa protein homologous to PsaA, a lipoprotein belonging to the LraI family and implicated in the cell adherence of Streptococcus pneumoniae and related species. By constructing a mutant of L. monocytogenes in which lpeA is deleted (lpeA mutant), we show that the PsaA-like protein LpeA is not involved in bacterial adherence but is required for entry of L. monocytogenes in eukaryotic cells. In contrast to wild-type bacteria, mutant bacteria failed to invade the epithelial Caco-2 and hepatocyte TIB73 cell lines, as confirmed by confocal microscopy. The mutant bacteria rapidly penetrated in mouse bone marrow-derived macrophages. Surprisingly, lpeA mutant bacteria survive better in macrophages than do wild-type bacteria. This was correlated with a weak exacerbation of virulence of the lpeA mutant in the mouse. LpeA is therefore a novel invasin favoring the entry of L. monocytogenes into nonprofessional phagocytes but not its invasion of macrophages. This is the first report of a lipoprotein promoting cell invasion of an intracellular pathogen.
The gram-positive, nonsporulating bacterium Listeria monocytogenes is a food-borne pathogen widely spread in the environment and responsible for severe infections in humans and most animals. The virulence of this facultative intracellular pathogen is due to its capacity to invade and multiply within host cells, including macrophages, hepatocytes, and epithelial and endothelial cells (51). During the intracellular infectious cycle of L. monocytogenes, bacteria first adhere to the surface of eukaryotic cells and subsequently penetrate into these cells, a process involving a zipper-type mechanism. Bacterial entry is a complex multistep molecular mechanism involving several eukaryotic receptors and bacterial ligands. The bacterial uptake by macrophages implicates receptors such as the C3bi and the C1q complement receptors and the macrophage scavenger receptor of phagocytic cells (1, 12, 16). Entry in nonprofessional phagocytes is mediated by the transmembrane glycoprotein E-cadherin (36), the gC1q-binding protein (7), and the Met receptor or hepatocyte growth factor (47). Other receptors including components of the extracellular matrix such as heparan sulfate proteoglycans (2) and fibronectin (22) have been described. The bacterial ligands are surface proteins behaving as adhesins or invasins. So far, several adhesins have been identified such as the autolysin Ami with a C-terminal domain similar to that of InlB (8, 35, 37), the 104-kDa Lap (45), and lectins (18, 40). The invasins such as the internalins InlA and InlB (20), the actin-polymerizing protein ActA (14, 29), and Iap (33) facilitate the process of penetration into eukaryotic cells. Invasion might be promoted through signaling cascades triggered by the interaction ligands-receptors, but the exact mechanisms are poorly understood. It is known that InlB is an agonist of the lipid phosphatidylinositol 3-kinase, the InlB-mediated uptake being associated with activation of the phosphatidylinositol 3-kinase (26, 27). The bacterial cell wall polymer lipoteichoic acid induces the expression of the proinflammatory cytokines interleukin 1α/β, interleukin 6, and tumor necrosis factor alpha in murine and human monocytes (4, 32). During the intimate contact of bacteria with endothelial cells, listeriolysine O and phospholipases can also elicit host cell responses, including the generation of lipid second messengers (48, 49), NF-κB activation (29, 46), stimulation of cytokines and chemokines, and induction of cell adhesion molecule expression (17, 24, 29). During invasion, Listeria bacteria are engulfed within phagocytic vacuoles and disrupt the phagosome membranes to be free in the cytoplasm. Listeriolysine O and phospholipases are involved in the escape from the phagosomal compartment. Through ActA, a bacterial membrane-anchored protein, intracytoplasmic bacteria become surrounded with actin filaments that form an actin tail allowing bacteria to move inside the cytoplasm and to spread from cell to cell (reviewed in reference 51).
In this work, we searched for new virulence factors implicated in the entry of L. monocytogenes into eukaryotic cells. We identified in silico from the recently completed sequence of the genome of L. monocytogenes (23) a gene encoding a PsaA-like protein belonging to the LraI family. PsaA is a lipoprotein previously implicated in the cell adherence of Streptococcus pneumoniae (44) and related species (10). By constructing a mutant in which this gene is deleted, we show that this PsaA-like protein is required for entry of L. monocytogenes into eukaryotic cells, not into macrophages. This protein, designated LpeA (for lipoprotein promoting entry), promotes entry and facilitates intracellular survival in infected macrophages.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
We used the reference strain of L. monocytogenes EGD-e belonging to serovar 1/2a (recently sequenced [23]) and the inlAB-defective mutant BUG8, previously described (20). Brain heart infusion (BHI) (Difco Laboratories, Detroit, Mich.) and Luria-Bertani (Difco) broth and agar were used as media in which to grow L. monocytogenes and Escherichia coli strains, respectively. We also used the following 17 wild-type Listeria strains previously described (5): L. monocytogenes (ATCC 19115, ATCC 19111, CNL880203, CHUT861141, CNL895793, CNL895795, INRA119, INRA85), L. ivanovii (ATCC 19119, SLCC2379), L. innocua (ATCC 33090, CHUT861158, INRA86), L. seeligeri (CHUT860478, CHUT861166, CHUT861167), and L. welshimeri (CHUT860477). Strains harboring plasmids were grown in the presence of the following antibiotics: for pCR derivates, kanamycin (Km) (50 μg/ml), and for pAUL-A derivates, erythromycin (Em) (150 μg/ml for E. coli and 5 μg/ml for L. monocytogenes). To analyze mutant bacteria, we studied 50 metabolic characters on API-50 strips (Biomerieux, Marcy l'Etoile, France). Bacterial growth assays were also performed with the previously defined synthetic medium F70 (41).
Genetic manipulations.
Chromosomal DNA, plasmid extraction, electrophoresis, restriction enzyme analysis, and amplification by PCR were performed according to standard protocols (43). Restriction enzymes and ligase were purchased from New England Biolabs and used as recommended by the manufacturer. DNA was amplified with the ampliTaq DNA polymerase of Thermus aquaticus from Perkin-Elmer (Branchburg, N.J.) in a Gene Amp System 9600 thermal cycler (Perkin-Elmer). Nucleotide sequencing was carried out with Taq DiDeoxy terminators and by the DyePrimer cycling sequence protocol developed by Applied Biosystems (Perkin-Elmer) with fluorescently labeled dideoxynucleotides and primers, respectively (Life Technologies). Labeled extension products were analyzed on an ABI Prism 310 apparatus (Applied Biosystems).
Construction of a mutant of L. monocytogenes lacking the lpeA gene
An lpeA mutant was constructed by deletion of a 270-bp internal fragment of lpeA and insertion of a promoterless aphA-3 gene conferring resistance to Km by double recombination, as previously described (6). The deletion replacement mutant of lpeA was constructed by inserting a 1,067-bp EcoRI-BamHI EGD DNA fragment (−38 to +1,017), an 855-bp BamHI Enterococcus faecalis DNA fragment carrying aphA-3, and a 1,062-bp BamHI-HindIII EGD DNA fragment (+1,293 to +2,336) between the EcoRI and HindIII sites of the thermosensitive shuttle vector pAUL-A, as previously described (6), to yield pAUL-lpeA ΩaphA3. These three DNA fragments were amplified by PCR from L. monocytogenes (EGD-e) genomic DNA by using the following primers pairs: papEco (5′-GGAATTCCGCAGCGGGGGTGTAAGAGTTGTTGTTTTTA-3′) and papBamas (5′-CGCGGATCCACGCCAACCAGGGGTACAATAC-3′); km1 (5′-CGGGATCCCGACTAACTAGGAGGAATA-3′) and km2 (5′-CGGGATCCCGGGTCATTATTCCCTCC-3′); and papBams (5′-CTTGTAAAAGCGGATCCAGACAATGCGGA-3′) and papHind (5′CCCAAGCTTGGGGGGGGCCTTTGGGACGGAGACAATTGCGGC-3′). Oligonucleotides were synthesized by Genset (Paris, France). The two amplified double-stranded DNA fragments were first cloned into the pCRTM cloning vector by using the TA cloningTM kit (Invitrogen Corporation, San Diego, Calif.). Plasmid pAUL-lpeA Ω aphA3 was electroporated into EGD, and transformants were selected for Em resistance at 30°C. Allelic exchange was obtained by homologous recombination by using a two-step procedure: at 40°C, a single crossing-over event integrated the entire plasmid into the chromosome; the plasmid was then excised by subculture at 30°C. The deletion was confirmed by PCR sequence analysis of chromosomal DNA from the mutant.
RNA isolation and Northern blot analysis.
Hybridizations of total RNA extracted during exponential phase at 37°C from wild-type EGD were done as described previously (41). A specific probe (length, 1,062 bp) used for hybridization was generated with the following primers pair: psa1 (5′-CGCACCGAAACAAGGCTTGCTATTTTC-3′) and pap2 (5′-CCGGGTTCGTAAAACGGAGCAAAAAC-3′).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis.
Proteins from extracts were prepared from cultures of bacteria grown in BHI broth at 37°C (optical density at 600 nm, 0.6). The bacterial pellets were suspended in 10 mM Tris-1 mM EDTA, and bacteria were disrupted with a Fastprep FP120 apparatus (BIO101; Ozyme) by three pulses of 30 s at a speed of 6.5 m/s. Bacterial debris were removed by centrifugation, and the resulting supernatant consisted of the cytoplasmic proteins. Electrophoresis and Western blotting were carried out as described previously in 10% sodium dodecyl sulfate-polyacrylamide minigels (Mini Protean II; Bio-Rad) (38). Nitrocellulose sheets were probed with anti-ScaA monoclonal antibody kindly provided by P. E. Kolenbrander (Bethesda) and antirabbit horseradish peroxidase-conjugated secondary antibody. Mouse monoclonal antibodies directed against InlA, InlB, or ActA obtained from P. Cossart (Institut Pasteur, Paris) were also used as previously described (38). Antibodies were used at a final dilution of 1:1,000. Antibody binding was revealed by adding 0.05% diaminobenzidine-tetrahydrochloride (Sigma) and 0.03% hydrogen peroxide (Sigma).
Infection of macrophages and cell lines.
Bone marrow-derived macrophages from C57/BL6 mice (IFFA-CREDO, Grenoble, France) were cultured and infected for growth curves at a cell-to-bacterium ratio of 1 to 1, as previously described (6). After 15 min of bacterial adherence on ice, macrophages were exposed for 15 min at 37°C (time zero). The numbers of intracellular bacteria were estimated in cell lysates at selected intervals (from time zero to 8 h postinfection). We also used the human colon carcinoma cell line Caco-2 (ATCC HTB37) and the murine embryonic hepatocyte cell line TIB73 (ATCC TIB73) from the American Type Culture Collection (Manassas, Va.). Cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing N-acetyl-l-alanyl-l-glutamine (Gibco Laboratories, Grand Island, N.Y.) and supplemented with 10% fetal bovine serum (Gibco Laboratories). Cells were maintained in 10% CO2 at 37°C without antibiotics. Cells were seeded at a density of 105/cm2 in 24-well tissue culture plates (Falcon Labware; Becton Dickinson & Co., Lincoln Park, N.J.). Monolayers were used 24 to 48 h after seeding. The invasion assays were carried out as described previously (37). Briefly, cells were inoculated with bacteria at a multiplicity of infection of approximately 100 bacteria/cell. They were incubated for 1 h and then washed three times with DMEM and overlaid with fresh DMEM containing gentamicin (10 mg/liter) to kill extracellular bacteria. At 2 and 4 h, cells were washed and lysed by addition of cold water. Viable bacteria released from the cells were plated onto BHI plates. Each experiment was carried out in triplicate and repeated three times, and results were expressed as means ± standard deviations. We compared the differences between the curves by Student's t test.
Confocal microscopy.
Infected cells were examined at progressive times by confocal microscopy. Double fluorescence labeling of F-actin and bacteria was performed as described previously (30) by using phalloidin coupled to Oregon Green 488 (Molecular Probes, Eugene, Oreg.) and a rabbit anti-Listeria serum (J. Rocourt, Institut Pasteur, Paris, France) revealed with an anti-immunoglobulin G antibody coupled to Alexa 546 (Molecular Probes). Images were scanned on a Zeiss LSM 510 confocal microscope.
Immunogold labeling.
Bacteria were grown overnight in BHI broth and processed as previously described (20). The grids were incubated for 1 h with rabbit anti-Listeria or rabbit anti-ScaA antibodies and further incubated with goat anti-rabbit immunoglobulin G conjugated to 10-nm gold particles.
Mouse virulence assay.
Six- to 8-week-old female Swiss mice (Janvier, Le Geneset St-Isle, France) were inoculated intravenously (i.v.) with various doses of bacteria. Mortality was monitored over a 14-day period for groups of five mice. The 50% lethal doses (LD50) were determined by the probit method. Bacterial growth in organs (spleen and liver) of mice infected i.v. with 105 bacteria was monitored as previously described (38). For growth in organs, we compared the data obtained with mutants to the data obtained with wild-type strains by a multifactorial variance analysis.
RESULTS
Identification of a gene encoding a PsaA-like protein in L. monocytogenes.
In a search for new virulence factors in L. monocytogenes, we used the sequence of the adhesin PsaA of S. pneumoniae to perform a Blast search (NCBI Blastp 2 version) of the entire sequence of the genome of L. monocytogenes EGD-e serovar 1/2a (23). We thus identified an ORF (open reading frame) encoding a PsaA-like protein of 310 residues (orf 3387.1), which we then designated lpeA. The deduced protein shares 50 to 60% identity with the sequences of various proteins encoding streptococcal adhesins, including PsaA of S. pneumoniae, FimA of Streptococcus parasanguis, ScaA of Streptococcus gordonii, SsaB of Streptococcus sanguis, and EfmA of Enterococcus faecium. These proteins constitute a group of streptococcal adhesins involved in adhesion to components of the oral cavity. All these proteins possessed a Leu-X-X-Cys sequence near the N-terminal end, corresponding to a lipoprotein consensus sequence and to the site for cleavage of the signal peptide (28). In addition, LpeA displays a metal-binding site formed by His-67, His-139, Glu-205, and Asp-280, as described for PsaA of S. pneumoniae (34).
By BLAST search, we also found a PsaA-like homologue in L. innocua, sharing 94% identity with LpeA (orf 3387.1) (23). Using primers specific for lpeA of L. monocytogenes, we showed by Southern blotting that these genes were present in all 17 tested strains of L. monocytogenes and other Listeria species (L. ivanovii, L. innocua, and L. seeligeri) (data not shown), suggesting that lpeA is highly conserved in the genus Listeria.
lpeA of L. monocytogenes belongs to an operon resembling those of a putative ATP-binding cassette (ABC) transporter family.
Analysis of the region of the L. monocytogenes genome comprising lpeA revealed the presence of three overlapping ORFs in the same orientation (orf 3390.3, orf 3389.1, and orf 3387.1), surrounded by two terminators (Fig. 1 A). The first orf encodes a putative ATP-binding protein of 240 amino acids, with a consensus nucleotide-binding site for ATP (GPNGAGKST) starting at position 33, corresponding to the consensus sequence (GXXGXGKS/T) in the glycine-rich loop of ATP-binding enzymes (52). In addition, a glutamine-glycine-rich motif (LSGGQLQR) could function as a peptide linker joining different domains of the protein. The second orf encodes a putative transmembrane protein of 279 residues, with a 16-amino-acid sequence (ALQTVGIILVVAMLITP) in position 182, also present in several hydrophobic membrane proteins involved in the transport of peptides and other small molecule transports (39).
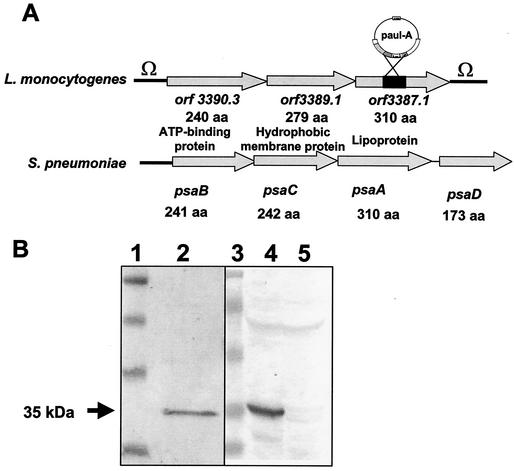
FIG. 1.
(A) Comparison of the genetic organization of the lpeA operon of L. monocytogenes with that of the psaA operon of S. pneumoniae. The lpeA operon is framed by two terminators (Ω). The lpeA gene is the third of the operon (orf3387.1), and there is no homologue for the regulator psaD in the genome of L. monocytogenes. lpeA encodes a lipoprotein homologous to PsaA of S. pneumoniae. Shown is the insertion of the promoterless aphA-3 cassette on plasmid pAUL-A into the lpeA gene. (B) Western blot analysis of the mutant of L. monocytogenes lacking lpeA. Bacterial extracts from EGD-e or lpeA mutant were tested with a monoclonal antibody (MAb) anti-ScaA, a PsaA homologue of S. gordonii. (Left panel) The MAb recognizes a 35-kDa band from a bacterial extract of S. gordonii. (Right panel) The MAb recognizes a 35-kDa band in wild-type EGD-e and does not react against the extracts from the lpeA mutant of L. monocytogenes. Lanes: 1 and 3, molecular markers; 2, S. gordonii; 4, EGD-e; 5, lpeA mutant.
A transcriptional analysis of the locus was performed by Northern blotting during exponential growth of L. monocytogenes in BHI medium. With an lpeA-specific probe, we detected by Northern blotting a single 2.3-kb transcript in the wild-type strain (data not shown), suggesting that lpeA is part of an operon of three genes. The genetic organization of the lpeA operon is similar to that of S. pneumoniae (Fig. 1A) and of the other psaA-like operons of E. faecium, E. faecalis, S. gordonii, S. sanguis, and S. parasanguis. However, we did not find a psaD-like regulator gene, which is usually located downstream pleA, in contrast to the other operons described. This gene was absent in the genome of L. monocytogenes, suggesting that the regulation of the lpeA operon notably differs from that of the other gram-positive species. This organization is reminiscent of that of the ABC-lipoprotein-dependent transporter systems, which is similar to the periplasmic binding-protein-dependent transport systems of gram-negative bacteria (bacterial permeases) (11).
Construction and phenotypic analysis of an lpeA mutant of L. monocytogenes.
We constructed an lpeA mutant of L. monocytogenes strain EGD-e by deletion of an internal fragment of lpeA gene (270 bp) and chromosomal integration by allelic replacement (EGDΔlpeA). Inactivation of lpeA was confirmed by Western blot analysis of bacterial extracts from the wild-type EGD-e or from the lpeA mutant by using antibodies directed against ScaA of S. gordonii, a protein sharing 57% of peptide identity with LpeA of L. monocytogenes. We found that this antiserum recognizes LpeA as a single 35-kDa band in bacterial extracts from the wild-type strain, in contrast to the mutant (Fig. 1B).
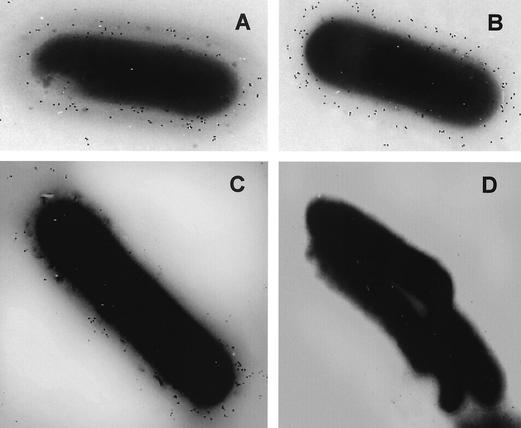
We did not find any difference between the mutant and the wild-type strain EGD-e with respect to microscopic morphology, motility, colony aspect, hemolysis on blood agar plates, metabolic profiles on API strips, and growth in BHI broth at 4, 37, or 42°C (data not shown). In contrast to previous data on the phenotype of a psaA mutant of S. pneumoniae (13), growth of the lpeA mutant of L. monocytogenes was not impaired on previously defined synthetic medium F70 (41). Since LpeA might be a putative factor involved in bacterial invasion, we confirmed by Western blot analysis that the proteins InlA, InlB, and ActA were produced at the same levels in the mutant and the wild-type bacteria (data not shown). Using the anti-ScaA serum, we also detected LpeA on the bacterial surface of the wild-type strain by immunogold labeling, in contrast to the absence of such labeling with the lpeA mutant, showing that LpeA is a surface-exposed protein (Fig. 2).
FIG. 2.
Immunogold labeling of the wild-type strain (A and C) and the lpeA mutant (B and D) of L. monocytogenes, using as control a polyclonal rabbit anti-Listeria antiserum (A and B) and a monoclonal anti-ScaA from S. gordonii directed against LpeA (C and D). Both strains are strongly labeled by the polyclonal anti-Listeria serum. Only the wild-type bacteria were labeled by the MAb anti-ScaA, contrasting with the absence of labeling of the lpeA mutant.
LpeA promotes cell invasiveness of L. monocytogenes.
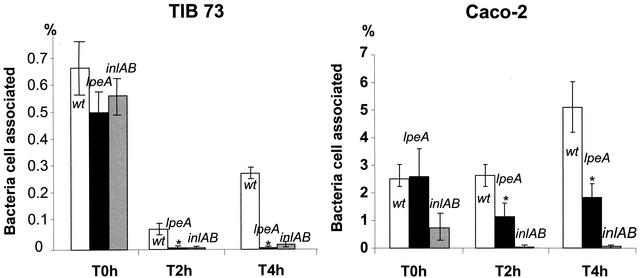
We then studied the capacity of the lpeA mutant to penetrate into and to replicate within cells in vitro. Using a multiplicity of infection of 100 bacteria/cell, we tested two different types of cell lines: the human enterocyte-like cell line Caco-2 and the murine embryonic hepatocyte cell line TIB73, which have been extensively used to study the in vitro infection by L. monocytogenes (15). Cell lines were exposed to strain EGD-e, EGDΔlpeA, or an inlAB mutant as a control for invasiveness. Cells were then washed and incubated in the presence of gentamicin (10 mg/liter) to eliminate extracellular bacteria. Bacteria were counted at time zero and after 2 and 4 h of incubation. No significant difference between EGD-e and EGDΔlpeA was observed by time zero in either cell line, indicating that LpeA does not influence adherence to cells (Fig. 3). As previously described (21), the rate of infection of Caco-2 cells by wild-type bacteria reached about 5% after 4 h, compared to a low infection rate of TIB73 cells (<0.3%) exposed to the same conditions. We found that the invasion by the lpeA mutant was significantly reduced in both cell lines, compared to that by wild-type EGD-e (P < 0.01; Student's t test). This defect of invasion was less pronounced in Caco-2 cells than that of the inlAB mutant (Fig. 3).
FIG. 3.
Role of LpeA in the entry of L. monocytogenes in epithelial and hepatocyte cell lines. TIB73 or Caco-2 cells were exposed to 100 bacteria of EGD-e, inlAB mutant (control), or lpeA mutant per cell. The initial numbers of bacteria associated to cells were similar at time zero for wild-type and lpeA mutant in both cell lines. As expected, the percentage of cells infected by wild-type bacteria after 2 and 4 h of incubation was significantly higher in Caco-2 than in TIB73 cells. The invasive capacity of both mutants was strongly impaired. Compared to that of wild-type bacteria, the invasion rate of the lpeA mutant was significantly reduced, although to a lesser extent than that of the inlAB mutant. Experiments were repeated three times for both cell lines. *, P < 0.01 by Student's t test.
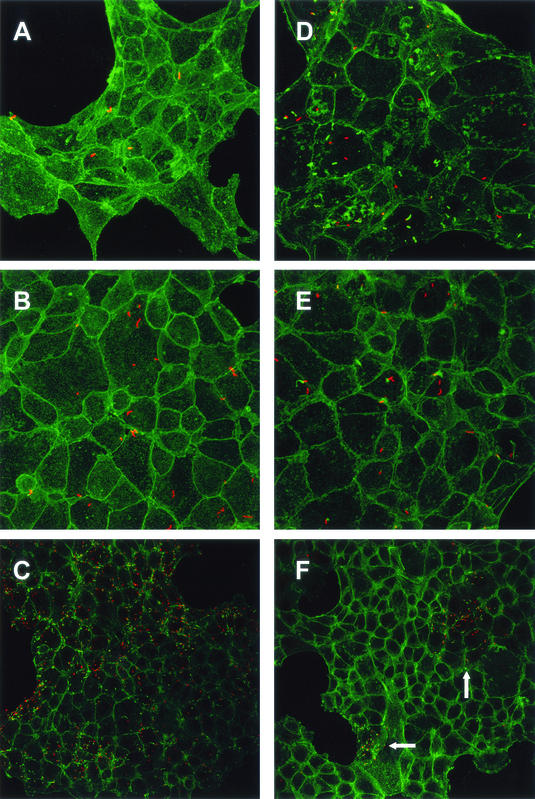
These results were confirmed by confocal electron microscopy of TIB73 cells exposed to bacteria under the same conditions (Fig. 4). By time zero, the numbers of cell-associated bacteria were similar for both strains (Fig. 4A and D). After 2 and 4 h of incubation, wild-type bacteria formed actin comets and massively invaded the cell monolayers as expected (Fig. 4B and C). In contrast, by time 2 h, most mutant bacteria remained located inside the phagosomal compartment without forming comets (Fig. 4E). After 4 h of incubation, the majority of TIB73 cells remained noninfected. However, we observed rare clusters of spreading mutant bacteria with polymerized actin (Fig. 4F). These results clearly indicate that LpeA is involved in cell invasion of L. monocytogenes.
FIG. 4.
Confocal microscopy of TIB-73 cells infected (100 bacteria/cell) with EGD-e (A, B, and C) or lpeA mutant (D, E, and F). Cells were observed at time zero (A and D) and 2 h (B and E) and 4 h (C and F) postinfection. F-actin was stained with phalloidin (green). Bacteria were labeled with an anti-Listeria serum (red). At time zero, no difference between the two strains was observed. After 2 h, many wild-type bacteria polymerized actin and started forming comets (B), whereas the majority of mutant bacteria were not associated with actin (E). After 4 h, wild-type bacteria massively spread to the entire cell monolayers (C). In contrast, the cell monolayers remained poorly infected by the mutant, except for rare clusters shown in panel F (indicated by arrows), in which mutant bacteria polymerized actin and multiplied inside cells. Magnifications, ×480 (A, B, D, and E) and ×320 (C and F).
Intramacrophage growth and virulence of the lpeA mutant.
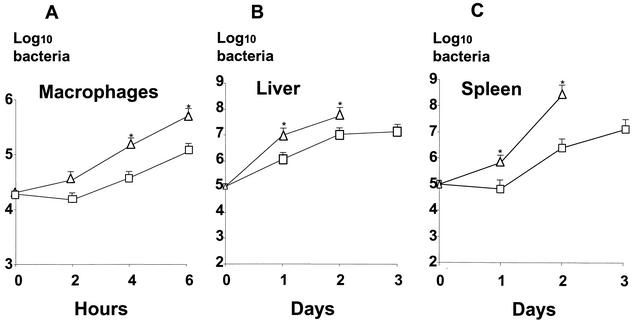
We then tested the capacities of lpeA mutant and of wild-type bacteria to survive in mouse bone marrow-derived macrophages. We observed that mutant lpeA bacteria invade and rapidly grow in macrophages. We repeatedly found that there was no initial drop of intramacrophage bacteria (2 h) for the mutant, in contrast to what was observed with wild-type bacteria (Fig. 5A). Then, both strains grew at a similar rate, with a higher final bacterial load for the lpeA mutant (Fig. 5A). This difference in growth between mutant and wild-type strains was statistically significant (P < 0.001).
FIG. 5.
Intramacrophage growth and virulence of the lpeA mutant of L. monocytogenes. □, wild-type EGD-e; ▵, lpeA mutant. (A) Growth of L. monocytogenes in bone marrow-derived macrophages from C57/BL6 mice. The cells were exposed for 15 min (time zero) to bacteria (1 bacterium per cell), and bacterial survival was monitored for 6 h after the infection. Entry was very efficient in both strains, and mutant bacteria repeatedly grew faster in macrophages (*, P < 0.001). (B and C) Growth of L. monocytogenes in the spleen (B) and the liver (C) of Swiss mice inoculated i.v. with 105 bacteria. Mutant bacteria grew faster in organs (*, P < 0.007) than did wild-type bacteria, and they induced an early mortality in mice by day 3.
Finally, we studied the role of LpeA in the virulence of L. monocytogenes by determining the LD50 after i.v. inoculation of Swiss mice. The LD50 of the mutant was estimated at 104.3, compared to 104.6 for the wild-type strain. After i.v. infection by the same inoculum (105 bacteria), the growth rate of mutant bacteria was greater in organs, especially in the spleen, than that of the wild-type bacteria, with an early mortality, beginning as soon as day 3 (Fig. 5B and C). This difference (days 1 to 2) was statistically significant (P < 0.007). Mice infected with wild-type bacteria died between days 4 and 6. These results indicate that the bacterial virulence of L. monocytogenes was weakly exacerbated in the absence of LpeA.
DISCUSSION
In this work, we identified in the genome of L. monocytogenes a previously unknown gene, lpeA, encoding a putative membrane lipoprotein homologous to PsaA, a structural adhesin of S. pneumoniae (42), and to other PsaA-like proteins produced by Streptococcus spp. implicated in cell adhesion and virulence of certain species (9, 19, 31). By transcriptional analysis, we found that lpeA is the third gene of an operon encoding an ATP-binding protein and a transmembrane protein homologous to several hydrophobic membrane proteins involved in the transport of peptides and other small-molecule transports (39). The genetic organization of the lpeA operon of L. monocytogenes is similar to that of the psaA operon of S. pneumoniae and reminiscent of that of the ABC lipoprotein-dependent transporter systems similar to the periplasmic-binding-protein-dependent transport systems of gram-negative bacteria (bacterial permeases) (10). As PsaA of S. pneumoniae, LpeA belongs to a family of surface-associated proteins designated lipoprotein receptor-associated antigen I (LraI), identified in at least seven species of Streptococcus and in two species of Enterococcus (3, 28). For example, they are implicated in the adherence of Streptococcus sanguis to platelet fibrin matrix (9), of S. pneumoniae to type II pneumocytes (3), of Streptococcus agalactiae to laminin (50), and of various Streptococcus species to salivary glycoproteins, fibrin, or epithelial cells (25), and also in the coaggregation of S. gordonii with Actinomyces (31). Some of them have been shown to be essential for the virulence of bacteria, such as S. pneumoniae, in experimental animal models. These polypeptides play a dual role in adhesion and transport. Indeed, the LraI polypeptides are components of ABC-type membrane transport systems implicated in the uptake of polypeptides, oligopeptides, and multiple sugars (10). Recently, it has been proposed that the members of the LraI family constitute a new family of extracellular solute-binding proteins specific for metal ions (Zn or Mn) (10, 39). The role of LpeA of L. monocytogenes as an ABC transporter remains unknown.
The main finding of this work is that LpeA is a surface-exposed protein promoting cell invasion by L. monocytogenes. Using a mutant lacking lpeA, we observed that LpeA-defective bacteria efficiently adhere to eukaryotic cells but fail to penetrate in vitro into epithelial and hepatocyte cells, as confirmed by confocal microscopy showing that very few lpeA mutant bacteria penetrate inside cells and then rapidly grow in the cytoplasm (Fig. 4). In contrast, an S. pneumoniae psaA mutant expresses a reduced adherence to A549 lung cells in vitro (3). These results demonstrate that (i) L. monocytogenes LpeA is not an adhesin and behaves as an invasin and that (ii) LpeA is not required to escape from the phagosomal compartment. This is the first demonstration that a putative lipoprotein promotes invasion by the pathogen L. monocytogenes.
The LpeA-dependent reduced invasion of epithelial and hepatocyte cell lines contrasts with the rapid invasion of bone marrow macrophages by lpeA mutant bacteria, resulting in a moderately higher bacterial intracellular load than with wild-type bacteria (Fig. 5A). This was confirmed by the finding of a moderate exacerbation of virulence in the absence of LpeA. Indeed, the mutant was fully virulent in the mouse with an LD50 estimated at 104.3, moderately lower than the LD50 value of the wild-type strain (104.6). Under the same infecting-challenge conditions, we observed higher bacterial loads in organs, associated with an early mortality (Fig. 5B and C). This result is surprising, since internalin-defective Listeria mutants express a weakly reduced level of virulence after i.v. inoculation (21). Moreover, an S. pneumoniae psaA mutant displays reduced virulence in mice infected by intranasal and intraperitoneal routes (3). Our results suggest that virulent bacteria at the initial phase of i.v. infection do not require invasins to directly spread in vivo from cell to cell. Circulating bacteria might mainly target resident or mobile macrophages, including dendritic cells, and then spread to adjacent cells, ultimately resulting in rapid growth in organs. This also suggests that direct invasion of hepatocytes or epithelial cells is not required for the expression of virulence in this model. To our knowledge, the exacerbation of virulence has never yet been observed with a deletion mutant of L. monocytogenes. The reasons for this virulence phenotype remain unknown. LpeA might act directly through a cell receptor or indirectly through its hypothetical function of the ABC transporter, which might influence the cell sensing or an intracellular cascade associated to cell entry through the uptake of polypeptides, oligopeptides, and multiple sugars. Our results clearly show that the putative lipoprotein LpeA of L. monocytogenes is a novel invasin involved in the entry process, but not in intracellular survival.
Acknowledgments
We kindly thank I. Dubail for technical assistance.
This work was supported by INSERM, The University of Paris V, two grants from the European Commission (contracts ERBCHRXCT 94-0451 and CT980036), and a grant from the DGA (no. 0034069).
Editor: J. D. Clements
REFERENCES
- 1.Alvarez-Dominguez, C., E. Carrasco-Marin, and F. Leyva-Cobian. 1993. Role of complement component C1q in phagocytosis of Listeria monocytogenes by murine macrophage-like cell line. Infect. Immun. 61:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Dominguez, F., J. A. Vazquez-Boland, E. Carrasco-Marin, P. Lopez-Mato, and F. Leyva-Cobian. 1997. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect. Immun. 65:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kDa putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borezée, E., T. Msadek, L. Durant, and P. Berche. 2000. Identification in Listeria monocytogenes of MecA, a homologue of the Bacillus subtilis competence regulatory protein. J. Bacteriol. 182:5931-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borezée, E., E. Pellegrini, and P. Berche. 2000. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect. Immun. 68:7069-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, L., B. Ghebrehiwet, and P. Cossart. 2000. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 19:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, L., H. Ohayon, and P. Cossart. 1998. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 9.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 63:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claverys, J. P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152:231-243. [DOI] [PubMed] [Google Scholar]
- 11.Claverys, J. R., C. Granadel, A. M. Berry, and J. C. Paton. 1999. Penicillin tolerance in Streptococcus pneumoniae, autolysis and the Psa ABC Mn-permease. Mol. Microbiol. 32:881-883. [DOI] [PubMed] [Google Scholar]
- 12.Croizé, J., J. Arvieux, P. Berche, and M. Colomb. 1993. Activation of the human complement alternative pathway by Listeria monocytogenes: evidence for direct binding and proteolysis of the C3 component on bacteria. Infect. Immun. 61:5134-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinthilhac, A., G. Alling, C. Granadel, and J. R. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: adc and psaA mutant exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-740. [DOI] [PubMed] [Google Scholar]
- 14.Domann, E., J. Wehland, M. Rohde, S. Pistor, M. Hartl, W. Goebel, M. Leimeister-Wächter, M. Wuenster, and T. Chakraborty. 1992. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 11:1981-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of InIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 16.Drevets, D. A., J. M. Leenen, and P. A. Campbell. 1993. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J. Immunol. 151:5431-5439. [PubMed] [Google Scholar]
- 17.Drevets, D. A. 1998. Listeria monocytogenes virulence factors that stimulate endothelial cells. Infect. Immun. 66:232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facinelli, B., E. Giovanetti, G. Magi, F. Biavasco, and P. E. Varaldo. 1998. Lectin reactivity and virulence among strains of Listeria monocytogenes determined in vitro using enterocyte-like cell line Caco-2. Microbiology 144:109-118. [DOI] [PubMed] [Google Scholar]
- 19.Fenno, J. C., A. Shaikh, G. Spatafora, and R. Fives-Taylor. 1995. The t7mA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol. Microbiol. 15:849-863. [DOI] [PubMed] [Google Scholar]
- 20.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard, J. L., F. Jaubert, and P. Berche. 1996. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J. Exp. Med. 183:359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilot, P., P. André, and J. Content. 1999. Listeria monocytogenes possesses adhesins for fibronectin. Infect. Immun. 67:6698-6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser, P., L. Frangeul, C. Buchriesser, A. Amend, F. Baquero Mochales, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chétaouni, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durand, O. Dusurget, K. D. Entian, H. Fsihi, F. Garcia Del-Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, J. Kreft, F. Junst, G. Kurapkat, E. Madueno, A. Maitournam, J. Mata Vicente, E. Ng, G. Nordsiek, B. de Pabolos, J. C. Perez-Dias, B. Remmel, M. Rose, C. Rusniok, T. Schluerer, N. Simoes, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 24.Greiffenberg, L., Z. Sokolovic, H. J. Schnittler, A. Spory, R. Böckmann, W. Goebel, and M. Kuhn. 1997. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol. Lett. 157:163-170. [DOI] [PubMed] [Google Scholar]
- 25.Harrington, D. J., J. S. Greated, N. Chanter, and I. C. Sutcliffe. 2000. Identification of lipoprotein homologues of pneumococcal PsaA in the equine pathogens Streptococcus equi and Streptococcus zooepidemicus. Infect. Immun. 68:6048-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 27.Ireton, K., B. Payrastre, and P. Cossart. 1999. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem. 274:17025-17032. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson, H. F. 1994. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121:133-140. [DOI] [PubMed] [Google Scholar]
- 29.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 30.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. Listeria monocytogenes induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 31.Kolenbrander, P. E., R. N. Andersen, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn, M., and W. Goebel. 1997. Responses by murine macrophages infected with Listeria monocytogenes crucial for the development of immunity to this pathogen. Immunol. Rev. 158:57-67. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn, M., and W. Goebel. 1989. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect. Immun. 57:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlan, A. M., and S. J. Foster. 1998. Molecular characterization of an autolytic amidase of Listeria monocytogenes EGD. Microbiology 144:1359-1367. [DOI] [PubMed] [Google Scholar]
- 36.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mège, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 37.Milohanic, E., R. Jonquières, P. Cossart, P. Berche, and J. L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eucaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 38.Nair, S., E. Milohanic, and P. Berche. 2000. The ClpC ATPase is required for cell adhesion and invasion of Listeria monocytogenes. Infect. Immun. 68:7061-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novak, R., J. S. Braun, E. Charpentier, and E. Tuomanen. 1998. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol. Microbiol. 29:1285-1296. [DOI] [PubMed] [Google Scholar]
- 40.Ofek, I., and N. Sharon. 1988. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect. Immun. 56:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouquette, C., R. Tascon, E. Pellegrini, J. M. Bolla, M. T. Ripio, J. A. Vazquez-Boland, and P. Berche. 1996. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol. Microbiol. 21:977-987. [DOI] [PubMed] [Google Scholar]
- 42.Russell, H., J. A. Tharpe, D. E. Wells, E. H. White, and J. E. Johnson. 1990. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J. Clin. Microbiol. 28:2191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Sampson, J. S., S. P. O'Connor, A. R. Stinson, J. A. Tharpe, and H. Russel. 1994. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect. Immun. 62:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santiago, N. I., A. Zipf, and A. K. Bhunia. 1999. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 65:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarzer, N., R. Nost, J. Seybold, S. K. Parida, O. Fuhrmann, M. Krüll, R. Schmidt, R. Newton, S. Hippenstiel, E. Domann, T. Chakraborty, and N. Suttorp. 1998. Two distinct phospholipases C of Listeria monocytogenes induce ceramide generation, nuclear factor-kB activation, and E-selectin expression in human endothelial cells. J. Immunol. 161:3010-3018. [PubMed] [Google Scholar]
- 47.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InlB-dependent internalization of Listeria is mediated by the met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 48.Sibelius, U., T. Chakraborty, B. Krögel, J. Wolf, F. Rose, R. Schmidt, J. Wehland, W. Seeger, and F. Grimminger. 1996. The listerial exotoxins listeriolysin and phosphatydilinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J. Immunol. 157:4055-4060. [PubMed] [Google Scholar]
- 49.Sibelius, U., F. Rose, T. Chakraborty, A. Darji, J. Wehland, S. Weiss, W. Seeger, and F. Grimminger. 1996. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect. Immun. 64:674-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lutticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the Lral adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehlan, and J. Kreft. 2001. Listeria pathogenesis and molecular determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase myosin kinases and other ATP requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]