Abstract
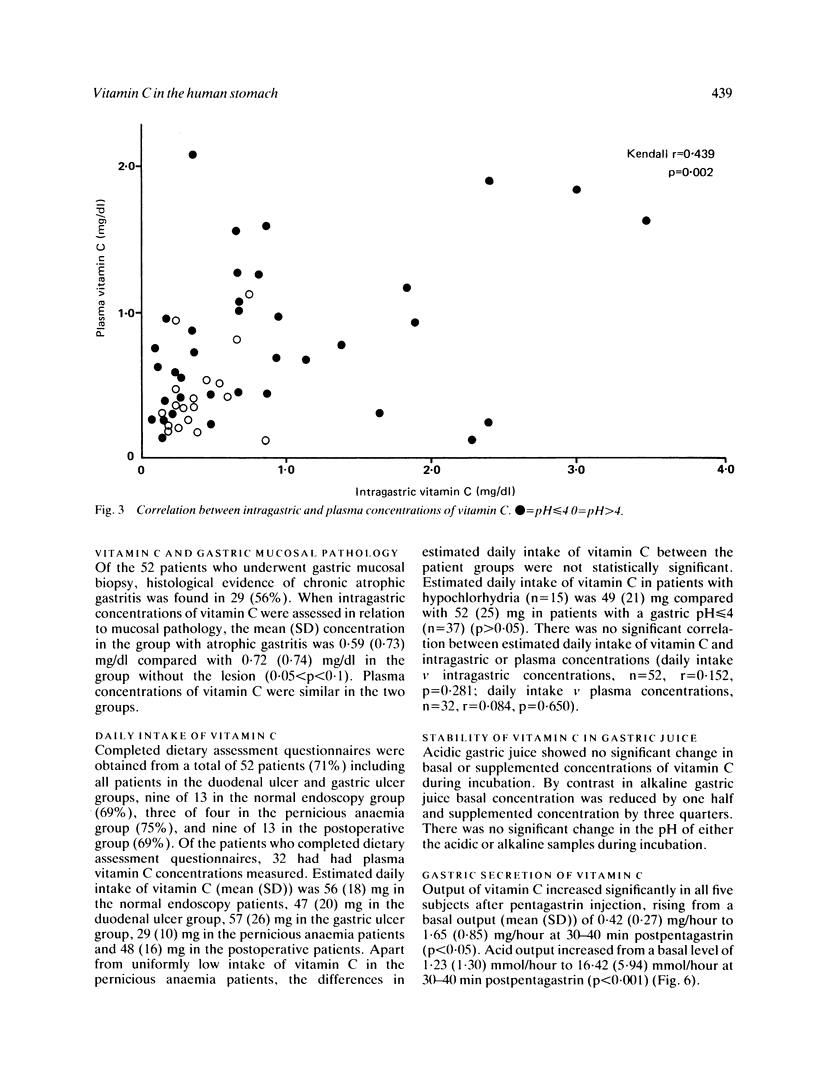
Fasting gastric juice pH and concentrations of vitamin C in gastric aspirate and plasma were measured in 73 patients undergoing endoscopy. Vitamin C concentrations were significantly lower in those with hypochlorhydria (pH greater than 4; n = 23) compared with those with pH less than or equal to 4 (p less than 0.005) and there was a significant correlation between gastric juice and plasma concentrations (p = 0.002). Patients with normal endoscopic findings had significantly higher intragastric concentrations of vitamin C than those with gastric cancer (p less than 0.001), pernicious anaemia (p less than 0.005), gastric ulcer (p less than 0.01), duodenal ulcer (p less than 0.05), or after gastric surgery (p less than 0.01). There was a strong trend (0.05 less than p less than 0.1) towards lower intragastric concentrations of vitamin C in patients with chronic atrophic gastritis. In vitro, vitamin C concentrations remained stable in acidic but fell significantly over 24 hours in alkaline gastric aspirate. Gastric secretory studies in five volunteers showed that vitamin C concentrations increased significantly after intramuscular pentagastrin. These findings suggest that the low fasting levels of vitamin C in hypochlorhydric gastric juice may be caused by chemical instability and that vitamin C may be secreted by the human stomach.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREIDENBACH A. W., RAY F. E. Gastric ascorbic acid in the gastritic guinea pig. Science. 1953 Nov 6;118(3071):557–557. doi: 10.1126/science.118.3071.557. [DOI] [PubMed] [Google Scholar]
- Bjelke E. Epidemiologic studies of cancer of the stomach, colon, and rectum; with special emphasis on the role of diet. Scand J Gastroenterol Suppl. 1974;31:1–235. [PubMed] [Google Scholar]
- Blackburn E. K., Callender S. T., Dacie J. V., Doll R., Girdwood R. H., Mollin D. L., Saracci R., Stafford J. L., Thompson R. B., Varadi S. Possible association between pernicious anaemia and leukaemia: a prospective study of 1,625 patients with a note on the very high incidence of stomach cancer. Int J Cancer. 1968 Jan 15;3(1):163–170. doi: 10.1002/ijc.2910030120. [DOI] [PubMed] [Google Scholar]
- Caygill C. P., Hill M. J., Kirkham J. S., Northfield T. C. Mortality from gastric cancer following gastric surgery for peptic ulcer. Lancet. 1986 Apr 26;1(8487):929–931. doi: 10.1016/s0140-6736(86)91041-x. [DOI] [PubMed] [Google Scholar]
- FREEMAN J. T., HAFKESBRING R. Comparative studies of ascorbic acid levels in gastric secretion and blood. III. Gastrointestinal diseases. Gastroenterology. 1957 May;32(5):878–886. [PubMed] [Google Scholar]
- Greenlaw R., Sheahan D. G., DeLuca V., Miller D., Myerson D., Myerson P. Gastroduodenitis. A broader concept of peptic ulcer disease. Dig Dis Sci. 1980 Sep;25(9):660–672. doi: 10.1007/BF01308325. [DOI] [PubMed] [Google Scholar]
- HAFKESBRING R., FREEMAN J. T. Comparative study of ascorbic acid levels in gastric secretion, blood, urine and saliva. II. Saturation studies. Am J Med Sci. 1952 Sep;224(3):324–328. doi: 10.1097/00000441-195209000-00013. [DOI] [PubMed] [Google Scholar]
- Haenszel W., Kurihara M., Locke F. B., Shimuzu K., Segi M. Stomach cancer in Japan. J Natl Cancer Inst. 1976 Feb;56(2):265–274. doi: 10.1093/jnci/56.2.265. [DOI] [PubMed] [Google Scholar]
- Haenszel W., Kurihara M., Segi M., Lee R. K. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972 Oct;49(4):969–988. [PubMed] [Google Scholar]
- Hole D. J., Quigley E. M., Gillis C. R., Watkinson G. Peptic ulcer and cancer: an examination of the relationship between chronic peptic ulcer and gastric carcinoma. Scand J Gastroenterol. 1987 Jan;22(1):17–23. doi: 10.3109/00365528708991850. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S., Wallcave L., Eagen M., Shubik P. Ascorbate-nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972 Jul 7;177(4043):65–68. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- Newill A., Habibzadeh N., Bishop N., Schorah C. J. Plasma levels of vitamin C components in normal and diabetic subjects. Ann Clin Biochem. 1984 Nov;21(Pt 6):488–490. doi: 10.1177/000456328402100608. [DOI] [PubMed] [Google Scholar]
- O'Connor H. J., Habibzedah N., Schorah C. J., Axon A. T., Riley S. E., Garner R. C. Effect of increased intake of vitamin C on the mutagenic activity of gastric juice and intragastric concentrations of ascorbic acid. Carcinogenesis. 1985 Nov;6(11):1675–1676. doi: 10.1093/carcin/6.11.1675. [DOI] [PubMed] [Google Scholar]
- Ohshima H., Bartsch H. Quantitative estimation of endogenous nitrosation in humans by monitoring N-nitrosoproline excreted in the urine. Cancer Res. 1981 Sep;41(9 Pt 1):3658–3662. [PubMed] [Google Scholar]
- Ruddell W. S., Bone E. S., Hill M. J., Walters C. L. Pathogenesis of gastric cancer in pernicious anaemia. Lancet. 1978 Mar 11;1(8063):521–523. doi: 10.1016/s0140-6736(78)90550-0. [DOI] [PubMed] [Google Scholar]


