Abstract
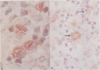
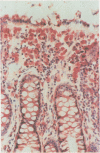
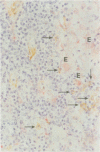
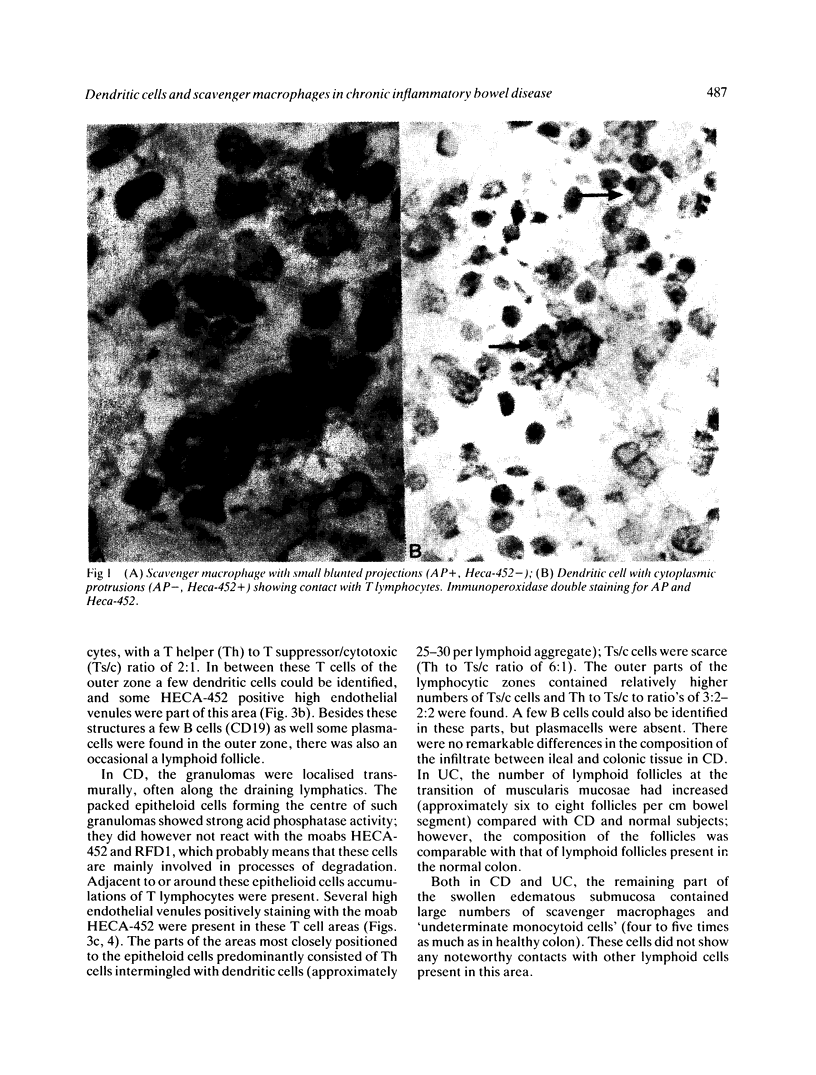
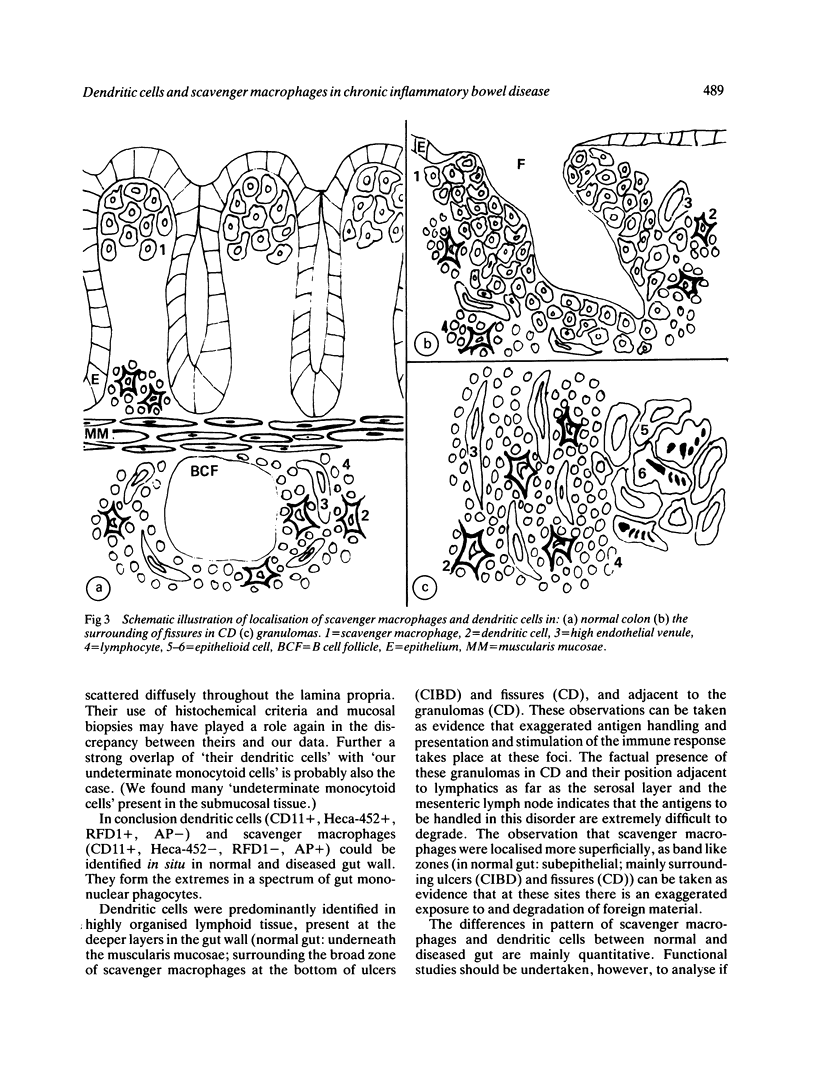
We used enzyme (acid phosphatase [AP]) and immunohistochemical techniques and a set of monoclonal antibodies (CD11, CD5, CD4, CD19, CD8, OKIa), including two recently developed antibodies--for example, HECA-452 (specific for an adhesion molecule on high endothelial venules) and RFD1 (specific for 'active' human dendritic cells) to analyse the composition of the gut wall infiltrate of 10 well defined cases of chronic inflammatory bowel disease (CIBD) (six Crohn's disease (CD), four ulcerative colitis (UC]. Two polar forms in a spectrum of gut mononuclear phagocyte types (CD11+) were identified: at the one extreme scavenger macrophages with blunted projections (AP+, Heca-452-, RFD1-) and at the other extreme, dendritic cells with long dendritic cytoplasmic projections (AP-, Heca-452+, RFD1+). Dendritic cells were mainly found in highly organised lymphoid tissue present at the deeper layers in the gut wall (normal gut: underneath the muscularis mucosae and T-cell areas of lymph follicles [25-30 per follicle]; surrounding the broad zone of scavenger macrophages at the bottom of ulcers (CIBD) and fissures (CD) and in the lymphoid aggregates [25-30 dendritic cells per aggregate] adjacent to granulomas (CD]. These observations can be taken as evidence that exaggerated antigen handling and presentation and stimulation of the immune response takes place at these foci. The observation that scavenger macrophages were localised more superficial, as band like zones (normal gut: subepithelial; mainly surrounding ulcers (CIBD) and fissures (CD] can be taken as evidence that at these spots the ingestion and degradation of foreign material takes place.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- Collings L. A., Waters M. F., Poulter L. W. The involvement of dendritic cells in the cutaneous lesions associated with tuberculoid and lepromatous leprosy. Clin Exp Immunol. 1985 Dec;62(3):458–467. [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A. M., Horst E., Pals S. T., Rouse B. N., Steere A. C., Picker L. J., Meijer C. J., Butcher E. C. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988 Jan;130(1):147–155. [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Jansen J., Falkenburg J. H., Stepan D. E., LeBien T. W. Removal of neoplastic cells from autologous bone marrow grafts with monoclonal antibodies. Semin Hematol. 1984 Jul;21(3):164–181. [PubMed] [Google Scholar]
- Korelitz B. I., Present D. H., Alpert L. I., Marshak R. H., Janowitz H. D. Recurrent regional ileitis after ileostomy and colectomy for granulomatous colitis. N Engl J Med. 1972 Jul 20;287(3):110–115. doi: 10.1056/NEJM197207202870302. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery T. D., Jr, Kraft S. C., Rothberg R. M. The influence of different techniques in characterizing human antibodies to cow's milk proteins. Clin Exp Immunol. 1972 Jun;11(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Mishra B. B., Poulter L. W., Janossy G., James D. G. The distribution of lymphoid and macrophage like cell subsets of sarcoid and Kveim granulomata: possible mechanism of negative PPD reaction in sarcoidosis. Clin Exp Immunol. 1983 Dec;54(3):705–715. [PMC free article] [PubMed] [Google Scholar]
- Morgan K. L. Johne's and Crohn's. Chronic inflammatory bowel diseases of infectious aetiology? Lancet. 1987 May 2;1(8540):1017–1019. doi: 10.1016/s0140-6736(87)92280-x. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Anderson K. C., Marti G., Bates M., Park E., Daley J. F., Schlossman S. F. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983 Jul;131(1):244–250. [PubMed] [Google Scholar]
- Perlmann P., Hammarström S., Lagercrantz R., Campbell D. Autoantibodies to colon in rats and human ulcerative colitis: cross reactivity with Escherichia coli O:14 antigen. Proc Soc Exp Biol Med. 1967 Jul;125(3):975–980. doi: 10.3181/00379727-125-32253. [DOI] [PubMed] [Google Scholar]
- Poulter L. W. Antigen presenting cells in situ: their identification and involvement in immunopathology. Clin Exp Immunol. 1983 Sep;53(3):513–520. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G., Seymour G. The involvement of interdigitating (antigen-presenting) cells in the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 1983 Feb;51(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Janossy G. The involvement of dendritic cells in chronic inflammatory disease. Scand J Immunol. 1985 May;21(5):401–407. doi: 10.1111/j.1365-3083.1985.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Thorbecke G. J., Baer R. L., Rosenthal S. A., Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976 Aug;25(2):137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- TAYLOR K. B., TRUELOVE S. C., WRIGHT R. SEROLOGIC REACTIONS TO GLUTEN AND COW'S MILK PROTEINS IN GASTROINTESTINAL DISEASE. Gastroenterology. 1964 Feb;46:99–108. [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilders M. M., Drexhage H. A., Kokjé M., Verspaget H. W., Meuwissen S. G. Veiled cells in chronic idiopathic inflammatory bowel disease. Clin Exp Immunol. 1984 Feb;55(2):377–387. [PMC free article] [PubMed] [Google Scholar]