Abstract
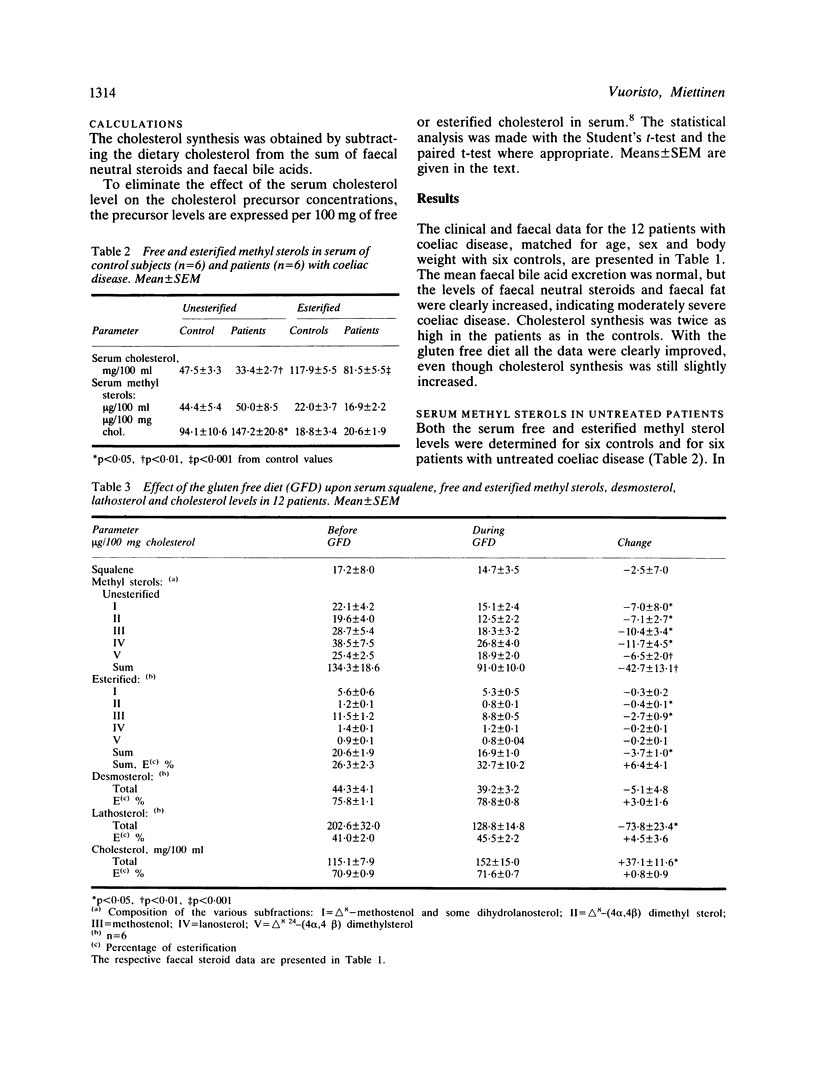
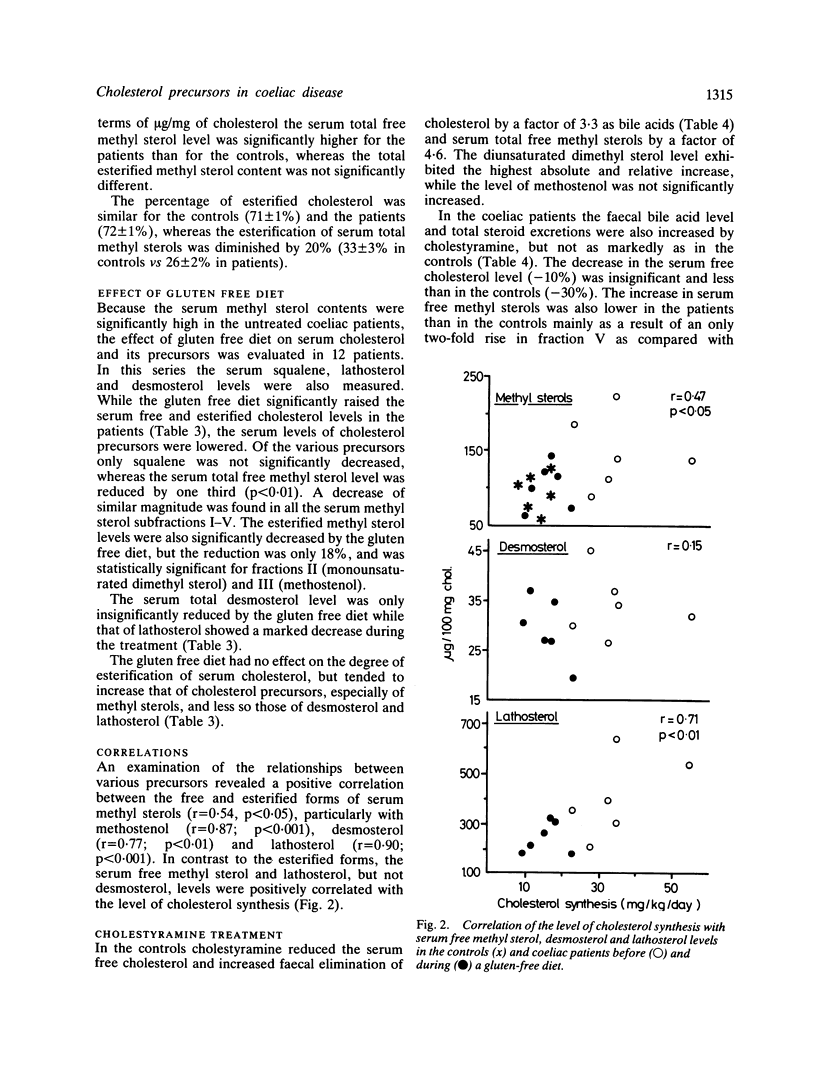
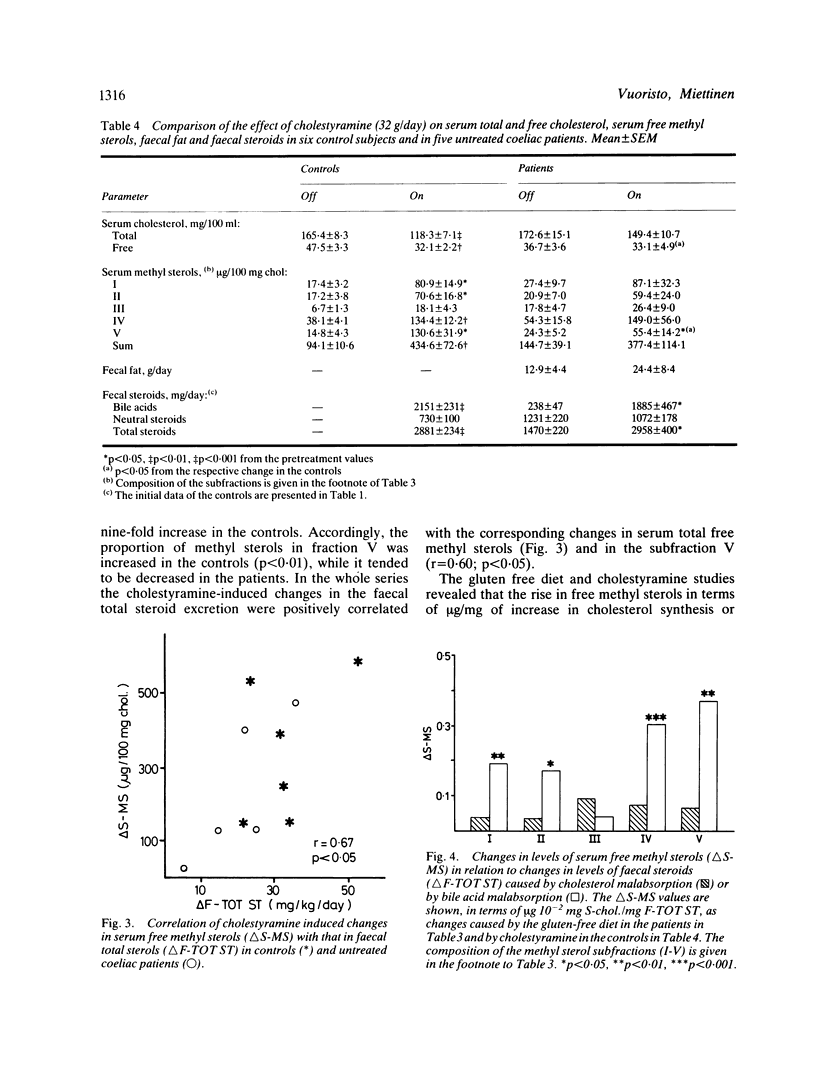
Enhanced biliary secretion and high faecal excretion of cholesterol are associated with increased cholesterol synthesis in coeliac disease. We have further investigated cholesterol synthesis in coeliac disease by determining the concentrations of faecal steroids and cholesterol precursors in serum, with and without a gluten free diet and while taking cholestyramine. The levels of unesterified methyl sterols and free and esterified lathosterol, but not those of squalene and desmosterol, were increased in proportion to the level of cholesterol synthesis, as measured with the sterol balance technique. Serum esterified methyl sterol concentrations were also slightly higher but, unlike free methyl sterols or lathosterol, they were not significantly correlated with cholesterol synthesis. The gluten free diet decreased the level of cholesterol synthesis, and the levels of lathosterol and free methyl sterols. There was less decrease in the concentration of esterified methyl sterols, and an insignificant decrease in the concentrations of squalene and desmosterol. Cholestyramine lowered the serum cholesterol concentration and increased that of serum free methyl sterols less in the patients than in the controls, and the increase was proportionate to increase of cholesterol elimination (or synthesis). The increase of serum free methyl sterols per unit of the increase of cholesterol elimination (or synthesis) was three times higher in the bile acid malabsorption caused by cholestyramine than in the cholesterol malabsorption caused by gluten enteropathy. On the other hand, the decrease in the level of serum cholesterol relative to the increase in cholesterol elimination (or synthesis) was higher in cholesterol malabsorption due to coeliac disease than in cholestyramine induced bile acid malabsorption. Effective secretion of newly synthesised and/or absorbed cholesterol directly into the bile could be a factor in the marked decrease of the serum cholesterol concentration in coeliac disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelin B. Cholesterol and bile acid metabolism in normo- and hyperlipoproteinaemia. Acta Med Scand Suppl. 1977;610:1–40. [PubMed] [Google Scholar]
- Brady D. R., Gaylor J. L. Enzymic formation of esters of methyl sterol precursors of cholesterol. J Lipid Res. 1971 May;12(3):270–276. [PubMed] [Google Scholar]
- Coyne M. J., Bonorris G. G., Goldstein L. I., Schoenfield L. J. Effect of chenodeoxycholic acid and phenobarbital on the rate-limiting enzymes of hepatic cholesterol and bile acid synthesis in patients with gallstones. J Lab Clin Med. 1976 Feb;87(2):281–291. [PubMed] [Google Scholar]
- Davignon J., Simmonds W. J., Ahrens E. H. Usefulness of chromic oxide as an internal standard for balance studies in formula-fed patients and for assessment of colonic function. J Clin Invest. 1968 Jan;47(1):127–138. doi: 10.1172/JCI105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Gamel W. G. Cholesterol synthesis in the intestine of man: regional differences and control mechanisms. J Clin Invest. 1971 Apr;50(4):872–880. doi: 10.1172/JCI106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- Erickson S. K., Shrewsbury M. A., Brooks C., Meyer D. J. Rat liver acyl-coenzyme A:cholesterol acyltransferase: its regulation in vivo and some of its properties in vitro. J Lipid Res. 1980 Sep;21(7):930–941. [PubMed] [Google Scholar]
- GLOMSET J. A., PARKER F., TJADEN M., WILLIAMS R. H. The esterification in vitro of free cholesterol in human and rat plasma. Biochim Biophys Acta. 1962 Apr 23;58:398–406. doi: 10.1016/0006-3002(62)90050-1. [DOI] [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Gould R. G., Swyryd E. A. Sites of control of hepatic cholesterol biosynthesis. J Lipid Res. 1966 Sep;7(5):698–707. [PubMed] [Google Scholar]
- Kesäniemi Y. A., Grundy S. M. Turnover of low density lipoproteins during inhibition of cholesterol absorption by neomycin. Arteriosclerosis. 1984 Jan-Feb;4(1):41–48. doi: 10.1161/01.atv.4.1.41. [DOI] [PubMed] [Google Scholar]
- Liu G. C., Ahrens E. H., Jr, Schreibman P. H., Crouse J. R. Measurement of squalene in human tissues and plasma: validation and application. J Lipid Res. 1976 Jan;17(1):38–45. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol precursors and their diurnal rhythm in lipoproteins of patients with jejuno-ileal bypass and ileal dysfunction. Metabolism. 1985 May;34(5):425–430. doi: 10.1016/0026-0495(85)90207-0. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A. Detection of changes in human cholesterol metabolism. Ann Clin Res. 1970 Dec;2(4):300–320. [PubMed] [Google Scholar]
- Miettinen T. A. Diurnal variation of cholesterol precursors squalene and methyl sterols in human plasma lipoproteins. J Lipid Res. 1982 Mar;23(3):466–473. [PubMed] [Google Scholar]
- Miettinen T. A. Effects of neomycin alone and in combination with cholestyramine on serum cholesterol and fecal steroids in hypercholesterolemic subjects. J Clin Invest. 1979 Nov;64(5):1485–1493. doi: 10.1172/JCI109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen T. A. Effects of neomycin alone and in combination with cholestyramine on serum methyl sterols and conversion of acetate and mevalonate to cholesterol. Scand J Clin Lab Invest. 1982 Apr;42(2):189–196. [PubMed] [Google Scholar]
- Miettinen T. A. Serum methyl sterols and their distribution between major lipoprotein fractions in different clinical conditions. Ann Clin Res. 1971 Oct;3(5):264–271. [PubMed] [Google Scholar]
- Miettinen T. A. Serum squalene and methyl sterols as indicators of cholesterol synthesis in vivo. Life Sci. 1969 Jul 15;8(14):713–721. doi: 10.1016/0024-3205(69)90007-1. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Kudchodkar B. Plasma squalene as an index of cholesterol synthesis. Clin Sci Mol Med. 1975 Dec;49(6):621–624. doi: 10.1042/cs0490621. [DOI] [PubMed] [Google Scholar]
- Nordby G., Norum K. R. Substrate specificity of lecithin:cholesterol acyltransferase. Esterification of desmosterol, b-sitosterol, and cholecalciferol in human plasma. Scand J Clin Lab Invest. 1975 Nov;35(7):677–682. [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Bicker S., Lawrie T. D., Morgan H. G. Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. N Engl J Med. 1980 May 29;302(22):1219–1222. doi: 10.1056/NEJM198005293022202. [DOI] [PubMed] [Google Scholar]
- Tavani D. M., Nes W. R., Billheimer J. T. The sterol substrate specificity of acyl CoA: :cholesterol acyltransferase from rat liver. J Lipid Res. 1982 Jul;23(5):774–781. [PubMed] [Google Scholar]
- Tilvis R. S., Miettinen T. A. A lack of esterification of lanosterol and other methyl sterols in human serum in vitro. Scand J Clin Lab Invest. 1980;40(7):671–674. doi: 10.1080/00365518009091980. [DOI] [PubMed] [Google Scholar]
- Vuoristo M., Miettinen T. A. Cholesterol absorption, elimination and synthesis in coeliac disease. Eur J Clin Invest. 1982 Aug;12(4):285–291. doi: 10.1111/j.1365-2362.1982.tb02234.x. [DOI] [PubMed] [Google Scholar]
- Vuoristo M., Miettinen T. A. Enhanced synthesis of cholesterol and its precursors in jejunal mucosa in coeliac disease. Gut. 1986 Apr;27(4):399–404. doi: 10.1136/gut.27.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoristo M., Miettinen T. A. Increased biliary lipid secretion in celiac disease. Gastroenterology. 1985 Jan;88(1 Pt 1):134–142. doi: 10.1016/s0016-5085(85)80145-1. [DOI] [PubMed] [Google Scholar]
- Vuoristo M., Tarpila S., Miettinen T. A. Serum lipids and fecal steroids in patients with celiac disease: effects of gluten-free diet and cholestyramine. Gastroenterology. 1980 Jun;78(6):1518–1525. [PubMed] [Google Scholar]


