Abstract
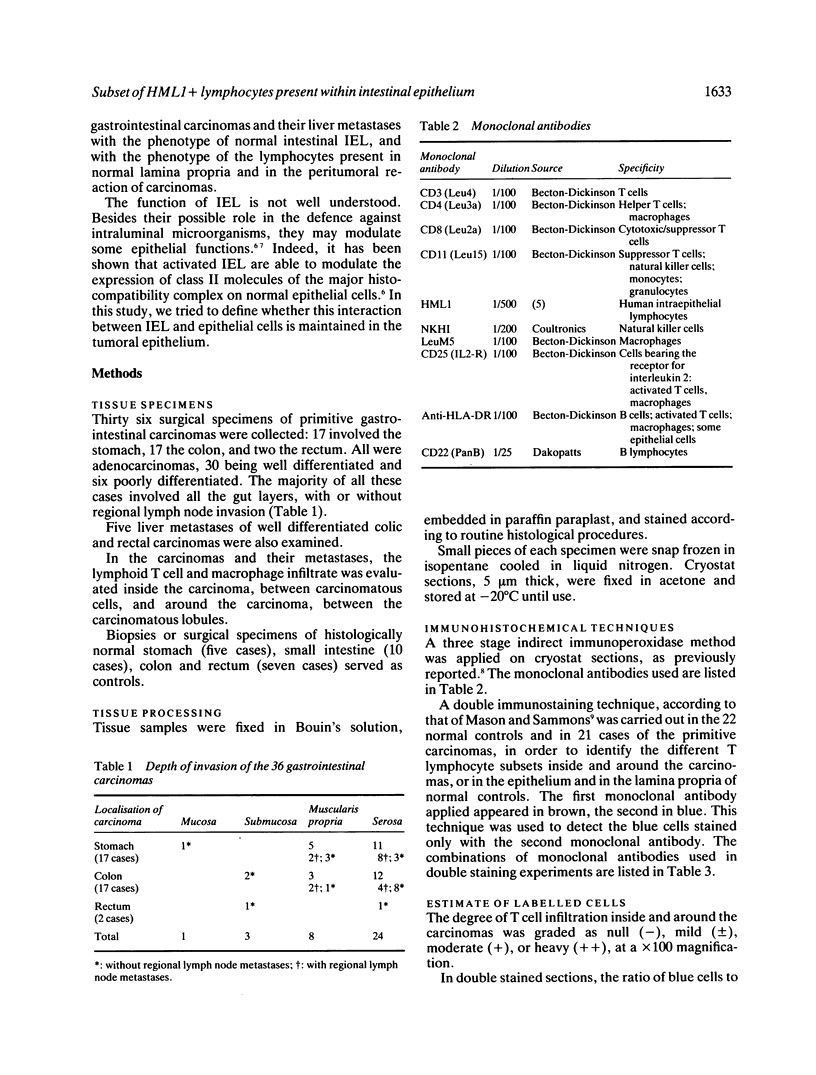
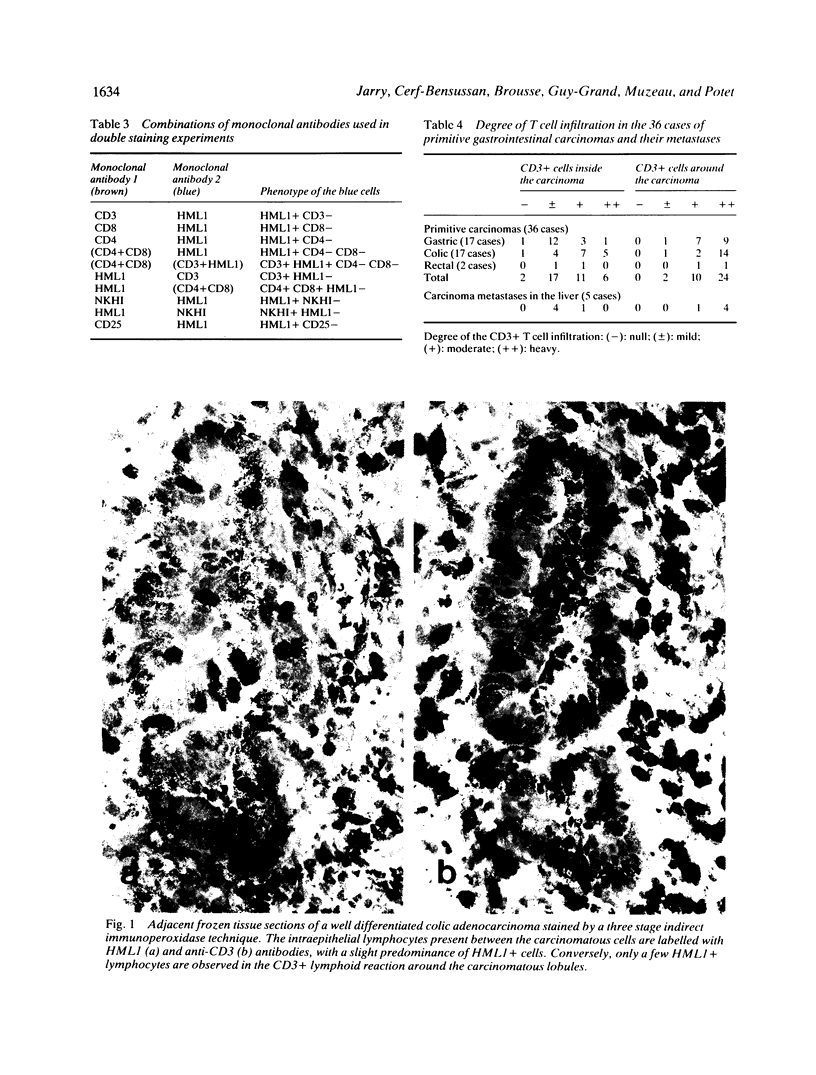
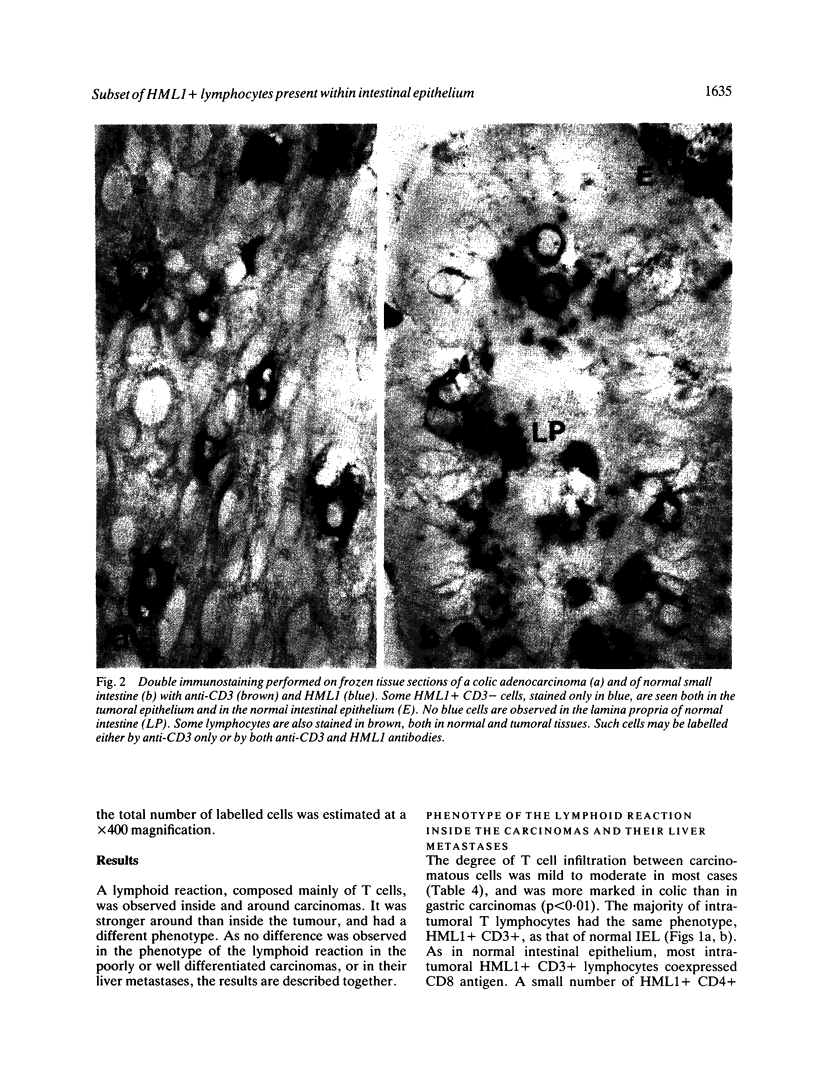
The present study shows that the distribution of T lymphocytes in gastrointestinal carcinomas and their metastases mimic the distribution of T lymphocytes in normal intestine. The composition of the peritumoral reaction resembled that of normal lamina propria with a predominance of CD3 + CD4 + T cells. In contrast, lymphocytes located between carcinomatous cells showed phenotypical features similar to those of intraepithelial lymphocytes (IEL) in normal intestine; in particu(abstractlar they expressed the antigen defined by HML-1, a monoclonal antibody raised against normal human intestinal IEL which reveals 95% IEL but very few cells in lymphoid (abstractorgans and blood. As normal intestinal IEL, the majority of intratumoral lymphocytes had the CD3+ CD8+ phenotype. A panel of monoclonal antibodies and double immunostaining techniques permitted a better characterisation of minor subsets of IEL. Two subsets of HML1 + CD3 + CD4- CD8- and of HML1+ CD3- cells, representing 2% and 3% of normal intestinal IEL respectively, did not significantly increase in carcinomatous epithelium. In contrast, in carcinomatous epithelium, but not in normal intestinal epithelium, we observed the appearance of a few lymphocytes displaying the phenotype of activated T cells (CD25+) or of natural killer cells (NKHI+) or of suppressor cells (CD11+). Such cells may participate in antitumoral defence. Although a similar population of HML1+ lymphocytes is associated with normal and carcinomatous intestinal epithelium, some interactions between lymphocytes and epithelial cells may not be maintained in tumoral epithelium. It has previously been shown that HLA-DR expression by enterocytes is modulated by intraepithelial lymphocytes. In our study, no correlation could be shown between the degree of lymphocytic infiltration and the expression of HLA-DR antigens on carcinomatous cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. A., Hogg N. Association of colorectal tumor epithelium expressing HLA-D/DR with CD8-positive T-cells and mononuclear phagocytes. Cancer Res. 1987 Jun 1;47(11):2919–2923. [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Gnéragbé T., Brousse N., Lisowska-Grospierre B., Griscelli C., Guy-Grand D. Monoclonal antibodies specific for intestinal lymphocytes. Monogr Allergy. 1988;24:167–172. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Dobbins W. O., 3rd Human intestinal intraepithelial lymphocytes. Gut. 1986 Aug;27(8):972–985. doi: 10.1136/gut.27.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977 Nov;18(11):921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry A., Brousse N., Souque A., Barge J., Molas G., Potet F. Lymphoid stromal reaction in gastrointestinal lymphomas: immunohistochemical study of 14 cases. J Clin Pathol. 1987 Jul;40(7):760–765. doi: 10.1136/jcp.40.7.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D., Goodall A., Scott B. B. T-lymphocyte populations in normal and coeliac small intestinal mucosa defined by monoclonal antibodies. Gut. 1986 Nov;27(11):1330–1337. doi: 10.1136/gut.27.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R. Ontogeny of the Thy-1-, Lyt-2+ murine intestinal intraepithelial lymphocyte. Characterization of a unique population of thymus-independent cytotoxic effector cells in the intestinal mucosa. J Exp Med. 1986 Jul 1;164(1):309–314. doi: 10.1084/jem.164.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Ruitenberg J. J., Phillips J. H. Human CD3+ T lymphocytes that express neither CD4 nor CD8 antigens. J Exp Med. 1986 Jul 1;164(1):339–344. doi: 10.1084/jem.164.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Sammons R. Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol. 1978 May;31(5):454–460. doi: 10.1136/jcp.31.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]