Abstract
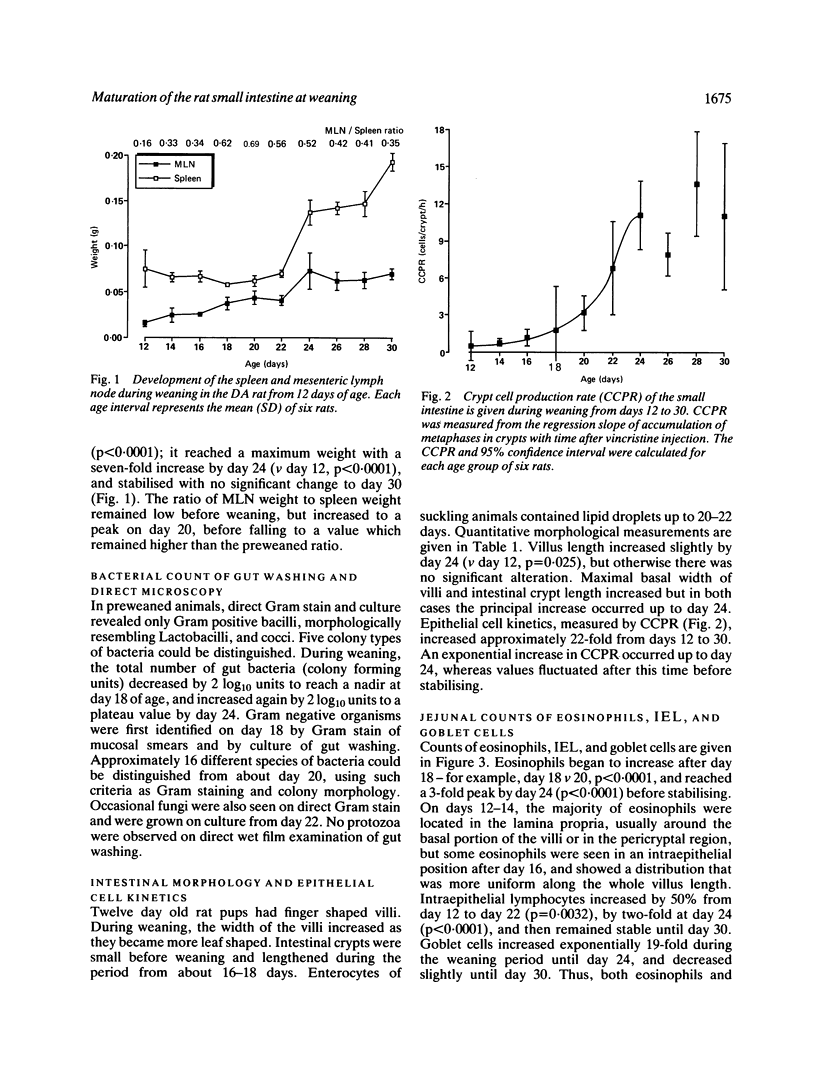
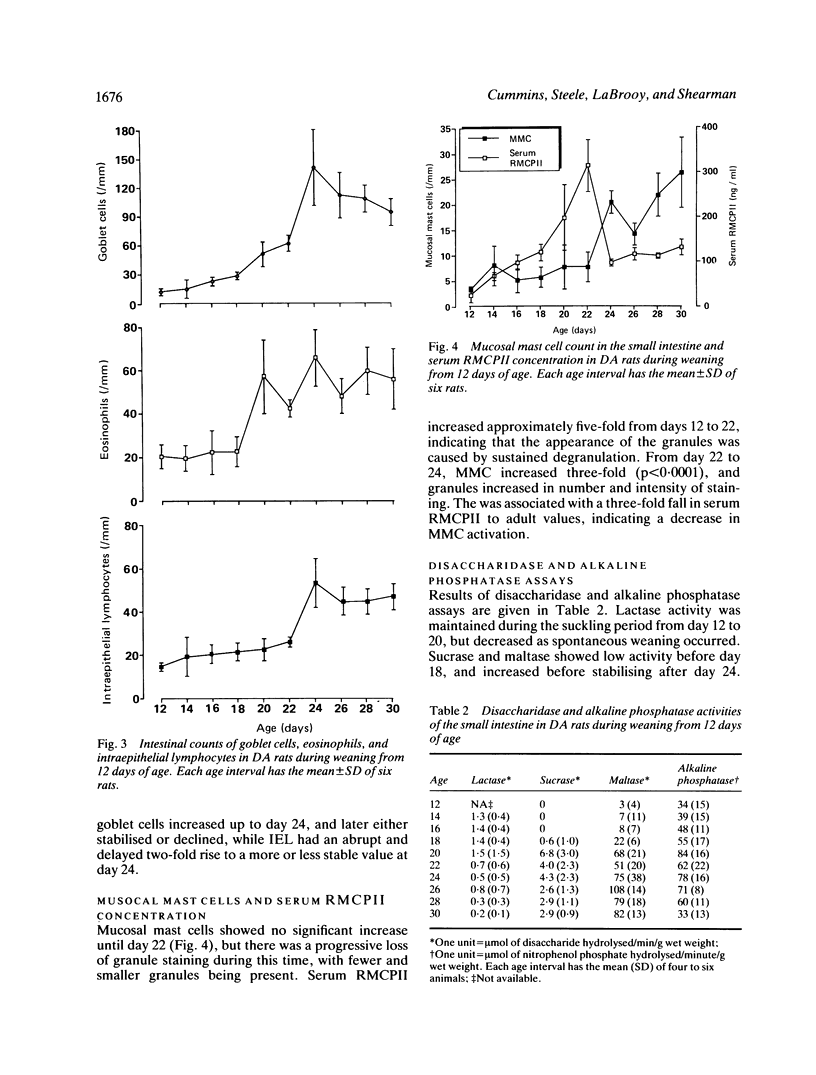
The relationship between maturation of the small intestine and change in mucosal immune activity was examined in the DA rat during the weaning period from 12 to 30 days. Two stages of jejunal maturation were observed: an initial stage of morphological development and crypt proliferation (days 12 to 22), followed by a period of stabilisation (days 24 to 30). By day 22 of the initial phase, villi increased principally in width but not in length, crypt length increased, and crypt cell production rate increased from 0.5 (day 12) to 11.1 (day 22) cells/crypt/hour. Various measures of mucosal immune activity showed a biphasic response. Up to days 20 to 22, the weight of the mesenteric lymph node increased seven-fold (p less than 0.0001), counts of jejunal eosinophils and goblet cells increased 3- (p less than 0.0001) and 19-fold (p less than 0.0001) respectively, and mean serum rat mucosal mast cell protease II, released from mucosal mast cells, increased from 24 (day 12) to 313 (day 22) ng/ml (p less than 0.0001). After day 22, mesenteric lymph node weight stabilised, eosinophil count stabilised and goblet cells decreased, serum rat mucosal mast cell protease II decreased three-fold (p less than 0.0001), and mean jejunal count of intraepithelial lymphocytes increased from 26 (day 22) to 54 (day 24) cells per mm of muscularis mucosae (p less than 0.0001), before stabilising. These results demonstrated a close association between maturation of the small intestine and change in activity of the mucosal immune system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nafussi A. I., Wright N. A. Cell kinetics in the mouse small intestine during immediate postnatal life. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;40(1):51–62. doi: 10.1007/BF02932850. [DOI] [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befus D. Intestinal mast cell polymorphism: new research directions and clinical implications. J Pediatr Gastroenterol Nutr. 1986 Jul-Aug;5(4):517–521. doi: 10.1097/00005176-198607000-00002. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- Cummins A. G., Kenny A. L., Duncombe V. M., Bolin T. D., Davis A. E. The effect of protein deficiency on systemic release of rat mucosal mast cell protease II during Nippostrongylus brasiliensis infection and following systemic anaphylaxis. Immunol Cell Biol. 1987 Aug;65(Pt 4):357–363. doi: 10.1038/icb.1987.40. [DOI] [PubMed] [Google Scholar]
- Cummins A. G., Munro G. H., Ferguson A. Effect of cyclosporin A on rat mucosal mast cells and the associated protease RMCPII. Clin Exp Immunol. 1988 Apr;72(1):136–140. [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Gerskowitch V. P., Russell R. I. Pre- and postweaning disaccharidase patterns in isografts of fetal mouse intestine. Gastroenterology. 1973 Feb;64(2):292–297. [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. Growth and development of "antigen-free" grafts of foetal mouse intestine. J Pathol. 1972 Feb;106(2):95–101. doi: 10.1002/path.1711060205. [DOI] [PubMed] [Google Scholar]
- Gibson S., Mackeller A., Newlands G. F., Miller H. R. Phenotypic expression of mast cell granule proteinases. Distribution of mast cell proteinases I and II in the rat digestive system. Immunology. 1987 Dec;62(4):621–627. [PMC free article] [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Sunshine P. Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res. 1969 Jan;3(1):27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Lebenthal E. Early weanling and precocious development of small intestine in rats: genetic, dietary or hormonal control. Pediatr Res. 1983 Aug;17(8):645–650. doi: 10.1203/00006450-198308000-00008. [DOI] [PubMed] [Google Scholar]
- Lund E. K., Bruce M. G., Smith M. W., Ferguson A. Selective effects of graft-versus-host reaction on disaccharidase expression by mouse jejunal enterocytes. Clin Sci (Lond) 1986 Aug;71(2):189–198. doi: 10.1042/cs0710189. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology. 1980 Sep;79(3):481–492. [PubMed] [Google Scholar]
- Mattingly J. A., Eardley D. D., Kemp J. D., Gershon R. K. Induction of suppressor cells in rat spleen: influence of microbial stimulation. J Immunol. 1979 Mar;122(3):787–790. [PubMed] [Google Scholar]
- Mayrhofer G., Fisher R. Mast cells in severely T-cell depleted rats and the response to infestation with Nippostrongylus brasiliensis. Immunology. 1979 May;37(1):145–155. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Whately R. J. Granular intraepithelial lymphocytes of the rat small intestine. I. Isolation, presence in T lymphocyte-deficient rats and bone marrow origin. Int Arch Allergy Appl Immunol. 1983;71(4):317–327. doi: 10.1159/000233414. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Nawa Y. Nippostrongylus brasiliensis: intestinal goblet-cell response in adoptively immunized rats. Exp Parasitol. 1979 Feb;47(1):81–90. doi: 10.1016/0014-4894(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V., Baca M. E. Experimental studies of immunologically mediated enteropathy. III. Severe and progressive enteropathy during a graft-versus-host reaction in athymic mice. Immunology. 1987 Jun;61(2):185–188. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Intraepithelial lymphocyte count and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982 Aug;83(2):417–423. [PubMed] [Google Scholar]
- Patrick M. K., Dunn I. J., Buret A., Miller H. R., Huntley J. F., Gibson S., Gall D. G. Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology. 1988 Jan;94(1):1–9. doi: 10.1016/0016-5085(88)90603-8. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Al-Nafussi A. The kinetics of villus cell populations in the mouse small intestine. II. Studies on growth control after death of proliferative cells induced by cytosine arabinoside, with special reference to negative feedback mechanisms. Cell Tissue Kinet. 1982 Nov;15(6):611–621. [PubMed] [Google Scholar]
- Wright N. A. The experimental analysis of changes in proliferative and morphological status in studies on the intestine. Scand J Gastroenterol Suppl. 1982;74:3–10. [PubMed] [Google Scholar]
- Younoszai M. K., Sapario R. S., Laughlin M. Maturation of jejunum and ileum in rats. Water and electrolyte transport during in vivo perfusion of hypertonic solutions. J Clin Invest. 1978 Aug;62(2):271–280. doi: 10.1172/JCI109126. [DOI] [PMC free article] [PubMed] [Google Scholar]


