Abstract
Serum bactericidal activity confers protection against meningococcal disease, but it is not known whether vaccine-induced anticapsular antibodies that lack bactericidal activity are protective. We developed an infant rat challenge model using a naturally occurring O-acetylated strain of Neisseria meningitidis group C and a strain that was negative for O acetylation (OAc). Rats 4 to 7 days of age inoculated intraperitoneally (i.p.) with ∼103 CFU of either strain developed >5 × 105 CFU/ml of blood obtained 18 h later. Dilutions of preimmunization sera given i.p. 2 h before the bacterial challenge had no effect on bacteremia, whereas group C anticapsular antibody in sera from adults immunized with meningococcal polysaccharide vaccine conferred complete or partial (>99% decrease in CFU per milliliter of blood) protection against the OAc-positive or OAc-negative strain, respectively, at antibody doses as low as 0.04 μg/rat. Anticapsular antibody at doses fivefold higher (0.18 to 0.2 μg/rat) in pooled sera from children immunized at a mean age of 2.6 years failed to protect rats, but antibody at the same or fivefold-lower dose in a serum pool from a group of children immunized at 4 years of age gave complete or partial protection. Protective activity was observed with some serum pools that lacked detectable complement-mediated bactericidal activity (titers < 1:4) and correlated with increasing antibody avidity. Thus, not only does the magnitude of the group C antibody response to meningococcal polysaccharide vaccine increase with increasing age but there are also age-related affects on antibody functional activity such that higher serum concentrations of vaccine-induced antibody are required for protection of immunized children than for immunized adults.
Neisseria meningitidis causes serious disease worldwide. In North America and Europe, the organism remains the most common cause of bacterial meningitis in children and young adults. Meningococci can be subdivided based on distinctive capsular polysaccharides. Isolates from five capsular groups—designated A, B, C, Y, and W135—are responsible for most cases of invasive disease. Thirty to forty percent of cases in North America and Europe are caused by capsular group C isolates.
The group C capsular polysaccharide consists of a homopolymer of α(2→9)-linked sialic acid (5, 31). In 90% of group C strains, the capsular polysaccharide is O acetylation (OAc) positive at positions 7 or 8, while in the remaining 10%, the capsular polysaccharide is OAc negative (3, 9). In OAc-positive strains, there is nonstoichiometric OAc of the capsular polysaccharide (thus, expression of both OAc-positive and OAc-negative epitopes) (31).
Meningococcal polysaccharide vaccines containing OAc-positive group C polysaccharide have been available for more than 30 years. These vaccines elicit serum antibodies largely in the absence of T-cell help (so-called thymic-independent antigens). In adults, these vaccines elicit high titers of group C complement-mediated serum bactericidal antibodies, and vaccination has been demonstrated to be highly effective in preventing disease (4, 11, 44). However, in infants and young children, the age groups at greatest risk of acquiring meningococcal disease, group C polysaccharide is poorly immunogenic (10, 34, 37) and is poorly efficacious (11, 48).
The presence of serum bactericidal activity remains the serologic hallmark of protective immunity against developing invasive meningococcal disease (6, 19). Although other immune mechanisms such as opsonic activity may also contribute to protection against meningococcal disease (12), it is not known whether sera that contain vaccine-induced anticapsular antibodies but lack complement-mediated bactericidal activity are protective against developing disease. To address this question, we developed an infant rat model of meningococcal bacteremia using either an OAc-positive or OAc-negative group C strain in order to correlate serum anticapsular and bactericidal antibody responses of children and adults vaccinated with meningococcal polysaccharide vaccine with the ability of the serum antibody to confer passive protection.
MATERIALS AND METHODS
Serum samples.
We utilized serum samples that had been collected during two previous immunogenicity studies in children (see below), or from 17 laboratory workers with occupational exposure to meningococci and who had been immunized with Menomune (50 μg of A, C, Y and W135 polysaccharide per 0.5-ml dose; Aventis Pasteur, Swiftwater, Pa.) by Employee Health at Children's Hospital and Research Center at Oakland. The samples were stored frozen at −70°C. The adults provided written informed consent, and use of these sera for the present study was approved by the Institutional Review Board (IRB) of Children's Hospital Oakland and Research Center at Oakland.
One collection of stored sera from immunized children came from a study performed in 1982 in an Amish community of genetic factors affecting immune responses to polysaccharide antigens (24). Written informed consent was obtained from the parents of all children, and the protocol was approved by the IRB of Washington University School Medicine, St. Louis, Mo. Subjects in this study were given separate intramuscular injections of Haemophilus influenzae type b (Hib) polysaccharide vaccine (5 μg; vaccine prepared at the University of Rochester, Rochester, N.Y.) and meningococcal polysaccharide vaccine (10 μg of A and C polysaccharides in 0.1 ml; vaccine prepared by Connaught Laboratories, Swiftwater, Pa. [now Aventis Pasteur]). Although the doses used were one-fifth of the usual doses of these vaccines, the lower doses were in the immunogenic range (16, 22). Blood samples were obtained immediately before vaccination and 1 and 2 months later. The Hib anticapsular antibody responses measured in this study have been previously reported (24, 25). Since only small volumes of sera remained from this study, the sera used in the present study were selected from 17 children, ages 1.3 to 7.0 years, based on the largest quantities of sera available.
The second collection of stored sera were from subjects, ages 6 months to 19.9 years, immunized with meningococcal polysaccharide vaccine (Menomune; Connaught Laboratories) in eastern Ontario, Canada, as part of control measures instituted by the regional health departments during an outbreak of group C disease in Canada. The serum samples were obtained under a protocol approved by the IRB of the Children's Hospital Eastern Ontario, Ottawa, Ontario, Canada, and written informed consent was obtained from all participants or parents. The group C immunogenicity results in a random sample of 345 participants have been reported (29). For the present study, pre- and postimmunization sera from 31 children, ages 4.1 to 9.9 years, were selected randomly.
Serology. (i) RABA.
The concentrations of group C anticapsular antibody were measured in the sera by a radioantigen binding assay (RABA) modified from that previously described (21). The group C meningococcal polysaccharide (provided by Aventis Pasteur) was derivatized with tyramine by a modification of the method of Lees et al. (30) and stored frozen in small portions at −70°C. On the week of the assay, an aliquot of antigen was thawed and the tyramine-polysaccharide was radiolabeled with 125I by the chloramine T method (21). The average specific activity of the iodinated derivatives was approximately 2.5 × 107 cpm/μg of polysaccharide. The RABA was performed in 1.5-ml microcentrifuge tubes containing 50 μl of test sera which had been diluted with phosphate-buffered saline containing 5% (vol/vol) fetal calf serum (PBS-FCS) and an equal volume of radiolabeled polysaccharide that had been diluted in PBS-FCS to contain antigen at ∼50 ng/ml (∼80,000 cpm/50 μl). After incubation at 37°C for 2 h, 100 μl of 25% polyethylene glycol 8000 in PBS-FCS was added to the reaction mixture, mixed briefly with a vortex, and incubated overnight at 4°C. The following day, the precipitated immunoglobulin and immune complexes containing bound 125I-labeled group C polysaccharide were harvested by centrifugation performed at 4°C for 10 min at 18,000 × g. The supernatant was removed, and the pellets were washed once in 0.5 ml of 12.5% polyethylene glycol 8000, and radioactivity in the precipitate were measured in a gamma counter. Antibody concentrations of the test sera were determined by comparison of the percentage of binding of the radiolabeled antigen by different dilutions of the test sample to that of a standard curve of binding at different dilutions of an anti-meningococcal serogroup A/C human reference serum (CDC1992, obtained from the National Institute for Biological Standards and Controls, Potters Bar, Hertfordshire, United Kingdom). This reference serum has been assigned a group C anticapsular antibody concentration of 32 μg/ml (27). As controls, each assay included a negative serum from an unimmunized adult with no detectable group C anticapsular antibody and three serum pools prepared from sera of immunized adults with low (0.4 μg/ml), medium (4.8 μg/ml), or high (44.8 μg/ml) anticapsular antibody concentrations.
All test serum samples were assayed in duplicate, and the respective pre- and postimmunization sera were measured in the same assay. For >95% of samples, bound polysaccharide in replicate dilutions of individual sera differed by less than 5%. To calculate interassay variation, the three serum pools described above were assayed on 12 occasions. The respective coefficients of variation for the results from these determinations ranged from 9.3 to 24%.
Anticapsular antibody avidity was measured in selected sera using a modification of the RABA originally described by Griswold et al. for measurement of Hib anticapsular antibody avidity (26). In brief, the total meningococcal group C antibody concentration was measured in each test serum by the RABA using radiolabeled group C polysaccharide at ∼83 ng/ml of instead of the usual ∼25 ng/ml in the final reaction vial. Also, incubation of serum and radiolabeled antigen was performed at 4°C for 18 h, instead of 37°C for 2 h as done in the usual RABA, described above. These changes in the protocol favored binding of both high- and low-avidity antibodies and provided a measurement of total antibody concentration, which in turn was used for calculation of the antibody avidity constant (Ka). The RABA was then repeated using a 25-fold-lower dose of radiolabeled antigen (3 ng/ml) to assure that the antibody concentration in the reaction vial was in excess, conditions that favor detection of primarily high-avidity antibodies. The fraction of bound antigen was determined at different antibody dilutions. The Ka was calculated by the following formula: Ka = fraction bound/{(1 − fraction bound) × [Ab]}, where fraction bound is the fraction of antigen bound at a particular antibody dilution, and [Ab] is the concentration of group C anticapsular antibody (molecular weight = 150,000) of the sample dilution tested, determined from the RABA using the high concentration of radiolabeled antigen (1). The avidity constant (nanomolar−1) for each sample was calculated from the average Ka determined at antibody dilutions giving binding between 20 and 80%. For each test sample, the Ka was measured on at least two to four separate occasions. The assigned Ka represented the average Ka, omitting outlier results in the calculation.
(ii) ELISA.
Immunoglobulin A (IgA), IgM, and IgG anticapsular antibody concentrations in serum were measured by an enzyme-linked immunosorbent assay (ELISA). The solid-phase antigen was adipic acid-derivatized polysaccharide, prepared from OAc-positive meningococcal C polysaccharide as previously described (23). The blocking buffer in the ELISA consisted of PBS-BSA (PBS with 1% [wt/vol] bovine serum albumin [BSA] [radioimmunoassay reagent grade; Sigma, St. Louis, Mo.]). Alkaline phosphatase-conjugated goat antibody specific for human IgA, IgM (Caltag Laboratories, Burlingame, Calif.), or IgG (Southern Biotechnology Associates, Inc., Birmingham, Ala.) antibodies were used as the secondary antibody detecting reagents. The ELISA employed replicate microtiter plates in which binding was measured using sera diluted with buffer alone or sera diluted with buffer containing soluble meningococcal group C polysaccharide (25 μg/ml) as an inhibitor. The antibody concentrations for the test sera were obtained by subtracting the respective absorbance values obtained with the sera diluted with the polysaccharide inhibitor from the corresponding values obtained with the samples diluted with buffer alone. The isotype-specific antibody concentrations in micrograms per milliliter were assigned to the test sera based on comparison to binding curves obtained with different dilutions of the CDC1992 reference serum, which has been assigned IgG, IgM, and IgA group C anticapsular antibody concentrations of 24.1, 2.0, and 5.9 μg/ml, respectively (27).
(iii) Inhibition of antibody binding by OAc-negative group C polysaccharide.
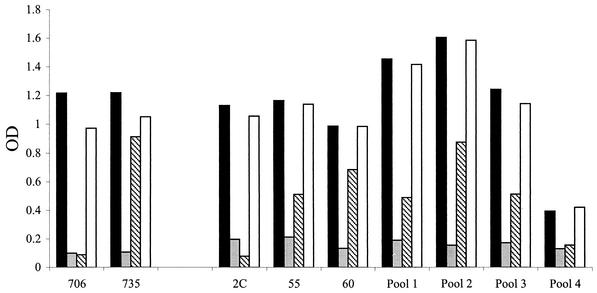
For determination of the proportion of group C anticapsular antibody that was specific for epitopes expressed by OAc-negative polysaccharide, the ELISA described above was modified such that dilutions of sera were added to four replicate microtiter plates. The solid-phase antigen was an adipic dihydrazide derivative of the OAc-positive polysaccharide (23) that, because of nonstoichiometric OAc of the capsular polysaccharide, expressed both OAc-positive and OAc-negative epitopes. The presence of both structures was confirmed by nuclear magnetic resonance spectroscopy performed by Neil Ravenscroft, University of Cape Town, Rondebosch, South Africa. In one plate, the serum samples were diluted with buffer alone. In the remaining three plates, the sera were diluted with an excess of soluble OAc-positive meningococcal group C or OAc-negative meningococcal group C or, as a negative control, meningococcal group Y polysaccharides, respectively. The concentrations of inhibitor used in different experiments ranged from 10 to 25 μg/ml (10- to 25-fold higher than that needed for ≥90% inhibition of specific binding). The results of a typical inhibition experiment are shown in Fig. 1. Positive controls in the assay include two murine monoclonal antibodies (MAbs), MAbs 706 and 735, with known preferential binding to OAc-negative or OAc-positive polysaccharide, respectively (14). (Antibodies were kindly provided by K. Stein, U.S. Food and Drug Administration, Bethesda, Md. [now at MacroGenics, Inc., Rockville, Md.]). These MAbs were assayed in parallel with the human sera except that the secondary antibody used to detect binding of the MAbs was specific for mouse Ig (heavy and light chain; Southern Biotech) instead of human IgG. Binding of MAb 706 was typically inhibited by >90% in the presence of soluble OAc-negative or OAc-positive polysaccharide, with the inhibition by the OAc-positive polysaccharide reflecting nonstoichiometric OAc. Binding of MAb 735 showed significant inhibition only in the presence of OAc-positive polysaccharide. As a second positive control, we measured inhibition of binding of a postimmunization serum (serum 2C), which was obtained from an adult immunized with a group C polysaccharide-tetanus toxoid conjugate prepared from de-O-acylated group C polysaccharide by Baxter Health Care (Columbia, Md.) (38) (serum kindly provided by Ray Borrow, Meningococcal Reference Laboratory, Manchester, United Kingdom). With this serum, we typically observed >90% inhibition with OAc-negative or OAc-positive polysaccharide (Fig. 1), a result consistent with nearly all of the group C antibodies in this serum being specific for OAc-negative epitopes. For calculation of percent inhibition in test samples, absorbance values in the plate containing serum samples diluted with the OAc-positive meningococcal group C polysaccharide inhibitor were considered to represent nonspecific background binding and, therefore, were subtracted as background binding from the absorbance values of the respective serum dilutions incubated in the plate with no inhibitor. The resulting specific total absorbance reflected antibody populations specific for both OAc-positive and -negative group C polysaccharide. The percent inhibition by the OAc-negative meningococcal group C polysaccharide or the negative control group Y polysaccharide inhibitor was calculated from the equation {[(total specific absorbance) − (inhibited absorbance)]/(total specific absorbance)} × 100, using serum dilutions where the total absorbance (optical density) was approximately 0.9 to 1.4. The percent inhibition was calculated from the mean results from a minimum of two to three experiments performed on separate days. The negative control meningococcal group Y polysaccharide gave no significant inhibition of antibody binding to group C polysaccharide (Fig. 1).
FIG. 1.
Binding to solid-phase adipic dihydrazide derivative of OAc-positive polysaccharide, which is known to express both OAc-positive and OAc-negative epitopes. Sera were tested in the presence of a 10-μg/ml concentration of soluble OAc-positive (gray bars) or OAc-negative (striped bars) meningococcal group C polysaccharide or, as a negative control, group Y polysaccharide (open bars) (solid bars, no polysaccharide). The control MAb 706 is known to recognize an epitope on OAc-negative polysaccharide, but its binding is inhibited by both OAc-negative and -positive group C polysaccharides because of nonstoichiometric OAc. MAb 735 preferentially binds OAc-positive polysaccharide. The inhibition observed in the ELISA is consistent with this binding specificity. Human serum 2C from an adult immunized with the Baxter group C conjugate vaccine prepared from OAc-negative polysaccharide is completely inhibited by OAc-negative polysaccharide.
(iv) Complement-dependent bactericidal antibody activity.
Bactericidal activity was measured as previously described (40) using log-phase test bacteria grown for approximately 2 to 2.5 h in Mueller-Hinton broth that had been supplemented with 0.25% glucose (wt/vol). Two group C meningococcal strains were tested—4243, which is naturally O-acetylated (OAc positive), and 4335, which is naturally not O-acetylated (OAc negative). These clinical isolates were provided by Trudy V. Murphy (now at Centers For Disease Control and Prevention [CDC], Atlanta, Ga.) and were collected during prospective surveillance of invasive meningococcal disease in Dallas County, Tex. (42). The two isolates were selected based on resistance to bactericidal activity of normal human serum and for their ability to cause bacteremia in infant rats (see below). The OAc status of these strains was determined by relative antibody binding as measured by a whole-bacterial-cell ELISA (40) using the OAc-positive specific murine MAb 1705.18, the OAc-negative specific MAb 181.1, and the backbone-specific MAb C2/1076.10 (14). The lack of OAc of the capsular polysaccharide purified from strain 4335 also was confirmed by nuclear magnetic resonance spectroscopy (kindly performed by Neil Ravenscroft). For measurement of bactericidal activity, all test sera were heated at 57°C for 30 min to inactivate intrinsic complement activity. The extrinsic complement source was serum from a healthy adult with no detectable anticapsular antibody to group C polysaccharide as tested by ELISA and no detectable intrinsic bactericidal activity against the target strains when tested at a final serum concentration of 20 or 40% (twofold higher than the serum concentration used for complement to test bactericidal activity in the test sera).
Passive protection against bacteremia in animal challenge model.
The ability of group C anticapsular antibodies to confer passive protection against N. meningitidis group C bacteremia was tested in infant rats challenged intraperitoneally (i.p.). In development of the animal challenge model, we tested 12 group C clinical isolates for their ability to cause bacteremia in infant rats; these strains had been passaged in the laboratory fewer than three times. Of these, strains 4243 and 4335 gave the most consistent bacteremia with challenge doses as low as 500 CFU/rat. Subsequently, each of these strains was serially passaged three times in infant rats and stored frozen at −80°C in skim milk. Bacterial challenge of the rats was performed as previously described for group B strains (40). In brief, 4- to 7-day-old pups from litters of outbred Wistar rats (Charles River, Raleigh, N.C.) were randomly redistributed to the nursing mothers. On the day before challenge, freshly thawed bacteria were inoculated onto chocolate agar and grown overnight at 37°C in 5% CO2. On the morning of the challenge, several colonies were inoculated into Mueller-Hinton broth supplemented with 0.25% glucose (wt/vol). After inoculation of the bacteria to a starting A620 of ∼0.1, the test organism was grown for approximately 2 h with shaking at 37°C in 5% CO2 to an A620 of ∼0.6. After washing the bacteria twice in PBS-BSA, the bacterial suspension was diluted in PBS-BSA to contain approximately 10,000 CFU/ml.
At time zero, groups of animals were treated i.p. with 100 μl of different dilutions of test or control sera. Two hours later the animals were challenged i.p. with 100 μl of bacteria (500 to 1,000 CFU/rat). Heparinized blood samples were obtained by cardiac puncture 18 h later, and quantitation of bacteremia was performed by directly plating aliquots of 100, 10, and 10 μl of a 1:10 dilution (i.e., 1 μl) of blood onto chocolate agar. The number of CFU per milliliter of blood was determined after overnight incubation of the plates at 37°C in 5% CO2. For calculation of geometric mean number of CFU per milliliter of blood, animals with sterile cultures were assigned a value of 1 CFU/ml, and animals whose samples produced a lawn of bacteria on culture plates streaked with 1 μl of blood were assumed to have more than 500,000 CFU/ml of blood and were assigned a value of 106 CFU/ml.
Statistical analysis.
For calculation of geometric means, the bactericidal titers and anticapsular antibody concentrations of each subject were logarithmically transformed (base 10). Titers or concentrations below the lower limit of detection in the assay were assigned as half of the lower limit. For each age group, the mean of the log values of the antibody titers or concentrations, and the associated standard error (SE) of the means are reported along with the respective geometric means. In the passive-protection experiments, the proportion of animals with bacteremia and the respective geometric means of the numbers of CFU per milliliter of blood were calculated for each group of animals. The significance of differences in the respective geometric means of the number of CFU per milliliter of blood from animals treated with different concentrations of group C anticapsular antibody were calculated using a two-tailed student t test (Excel software). For the purpose of determining the relationship between antibody avidity and passive protective activity, for each serum sample or serum pool, we determined the dose of antibody per infant rat required for a 2-log10 decrease in the number of CFU per milliliter of blood (i.e., 90% decrease), compared to that of control animals pretreated with preimmune serum or PBS. For the regression analyses, protective doses less than 0.008 μg/rat were assigned a value of 0.004 μg/rat and protective doses greater than 0.18 or 0.2 μg/rat (the highest doses tested for passive protection against the OAc-negative and -positive strains, respectively) were assigned a value of 0.4 μg/rat. The correlation coefficient, r, and the r2 values between the Ka of each serum or serum pool and the respective log10 of the protective antibody dose were calculated by analysis of variance regression (polynomial degree = 2) (GB-STAT, version 6.5; PPC, Dynamic Microsystem, Silver Spring, Md.).
RESULTS
Age-related anticapsular antibody response.
Table 1 summarizes the group C anticapsular antibody concentrations measured in serum samples obtained immediately before immunization and 1 to 2 months later. The results are stratified by the age of the subject at the time of immunization and the study in which the samples were obtained (groups 1 and 2 were from the Canadian study, and groups 3 and 4 were from the study done in an Amish community in Missouri). As expected (15, 37), the magnitude of the geometric mean serum anticapsular antibody concentrations increased with increasing age, being lowest in the 2-year-olds, intermediate in the 4-year-olds, and highest in 8- to 9-year-olds and adults. For all groups tested, IgG anticapsular antibody responses predominated with lesser concentrations of IgM anticapsular antibody.
TABLE 1.
Group C serum antibody responses of adults and children immunized with meningococcal polysaccharide vaccine
| Groupa | No. of subjects tested | Mean age (yr) (range) | Mean log antibody concn (μg/ml) ± SE (geometric mean) as determined by:
|
|||
|---|---|---|---|---|---|---|
| RABA with:
|
ELISA with postimmunization sera and:
|
|||||
| Preimmunization sera | Postimmunization sera | IgM | IgG | |||
| Adults | 17 | 34 (18-45) | −0.43 ± 0.12 (0.4) | 1.23 ± 0.11b (17.0) | 0.23 ± 0.16 (1.7) | 0.92 ± 0.20 (8.2) |
| Children | ||||||
| Group 1 | 16 | 9.5 (8.2-9.9) | −0.63 ± 0.18 (0.2) | 1.39 ± 0.14b,c (24.5) | −0.15 ± 0.25 (0.7) | 1.18 ± 0.20 (15.1) |
| Group 2 | 15 | 4.5 (4.1-4.9) | −1.53 ± 0.10 (<0.1) | 0.93 ± 0.07c,d (8.5) | −0.70 ± 0.32 (0.2) | 0.86 ± 0.08 (7.2) |
| Group 3 | 5 | 4.6 (2.4-7.0) | −0.67 ± 0.39 (0.2) | 1.03 ± 0.07e (10.8) | NDf | ND |
| Group 4 | 12 | 2.6 (1.3-4.8) | −1.39 ± 0.15 (<0.1) | 0.37 ± 0.04d,e (2.4) | ND | ND |
Groups 1 and 2 were children immunized in a study in Canada (29). Groups 3 and 4 were Amish children immunized in a study in the United States (24). Preimmunization sera were obtained immediately before vaccination. Post-immunization sera were obtained 1 to 2 months later. IgM, IgG and bactericidal antibody responses were not performed on individual sera from Groups 3 and 4 because of insufficient quantities of sera (See methods).
The geometric mean antibody concentrations of the adults and children in group 1 are not significantly different.
The geometric mean antibody concentrations of groups 1 and 2 are significantly different (P < 0.01).
The geometric mean antibody concentrations groups 2 and 4 are significantly different (P < 10−6).
The geometric mean antibody concentrations of groups 3 and 4 are significantly different (P < 10−4).
ND, not done because of insufficient quantities of sera.
Table 2 summarizes the serum bactericidal antibody responses measured against two group C test strains: 4243, which expresses O-acetylated capsular polysaccharide (OAc positive), and 4335, a strain that expresses capsular polysaccharide which is not O acetylated (OAc negative). The adults and the 8- to 9-year-old children showed significant increases in the geometric mean bactericidal titers after immunization. The respective postimmunization geometric mean titers of antibodies against the OAc-negative strain were approximately twofold higher than those measured with the OAc-positive strain. Three-quarters or more of the immunized adults or 8- to 9-year-old children had postimmunization bactericidal antibody titers of 1:4 or greater when tested against either group C strain. When measured with human complement, this threshold correlates with protection against developing meningococcal disease (6, 19, 45).
TABLE 2.
Group C serum bactericidal titers of adults and children immunized with meningococcal polysaccharide vaccine
| Groupa | No. of subjects tested | Mean age (yr) (range) | Reciprocal titer of bactericidal antibody in serum
|
|||||
|---|---|---|---|---|---|---|---|---|
| Strain 4243 (OAc positive)
|
Strain 4335 (OAc negative)
|
|||||||
| Mean log ± SE (geometric mean)
|
Post % ≥4c | Mean log ± SE (geometric mean)
|
Post % ≥4 | |||||
| Preimmunization | Postimmunization | Preimmunization | Postimmunization | |||||
| Adults | 17 | 34 (18-45) | 0.30 ± 0.00 (2.0) | 1.24 ± 0.17 (17.2) | 76 | 0.38 ± 0.06 (2.4) | 1.58 ± 0.22 (37.9) | 76 |
| Children | ||||||||
| Group 1 | 16 | 9.5 (8.2-9.9) | 0.42 ± 0.07 (2.7) | 1.14 ± 0.19 (13.7) | 75 | 0.47 ± 0.10 (3.0) | 1.48 ± 0.22 (30.1) | 88 |
| Group 2b | 15 | 4.5 (4.1-4.9) | 0.30 ± 0.00 (2.0) | 0.36 ± 0.04 (2.3) | 13 | 0.30 ± 0.00 (2.0) | 0.67 ± 0.14 (4.7) | 47 |
Groups 1 and 2 were children immunized in a study in Canada (29). Bactericidal titers were not obtained for individual sera from children in groups 3 and 4 because of insufficient quantities.
Children in group 2 had lower geometric mean bactericidal antibody responses than did the adults (P < 0.002) or children in group 1 (P < 0.004) for both the OAc-positive and OAc-negative strains.
Percentage of individuals with postimmune bactericidal titers of ≥1:4.
The bactericidal antibody responses of the children immunized at 4 years of age were much lower than those of the older children or adults. Only half of 4-year-olds developed titers of 1:4 or more when measured against the OAc-negative test strain, and only 13% developed titers of 1:4 or more against the OAc-positive strain. The low bactericidal antibody responses of the immunized 4-year-olds were unexpected since most of the children showed high anticapsular antibody responses to vaccination as measured by the RABA and/or IgG ELISA (Table 1), which used OAc-positive group C polysaccharide as the target antigen. The low or absent bactericidal responses despite the presence of relatively high serum anticapsular antibody concentrations imply that the anticapsular antibodies elicited by vaccination in these young children have poorer functional activity than those elicited by vaccination of older children or adults.
To investigate the protective activity of these antibodies in an animal challenge model, we pooled the respective pre- and postimmunization sera of children immunized at different ages (pre- and postimmunization pools 1, 2, 3, and 4 from groups 1, 2, 3, and 4, respectively, shown in Table 1). Table 3 summarizes the antibody concentrations measured in these serum pools and the results of characterization of the group C anticapsular antibody concentrations, isotype distributions, avidity, and the percentage of the anticapsular antibody response directed against the OAc-negative epitopes. For comparison, the corresponding results are shown for pre- and postimmunization serum samples from three representative control adults given meningococcal polysaccharide vaccine: subject 54, a low group C responder to vaccination, and subjects 55 and 60, who were chosen as representative of high responders to vaccination.
TABLE 3.
Group C anticapsular antibody concentrations of selected pre- and postimmunization sera or serum pools
| Subject or pool no. (mean age [yr]) | Total Anticapsular Antibody by RABA
|
Anticapsular Antibody by ELISA (postimmunization serum)
|
|||||
|---|---|---|---|---|---|---|---|
| Concn (μg/ml) in serum
|
Mean Ka ± SE of postimmunization serum (nM−1) | Concn (μg/ml) of:
|
% IgG inhibited by OAc-negative polysaccharideb | ||||
| Preimmunization | Postimmunization | IgM | IgA | IgG | |||
| Subjects | |||||||
| 54 (adult) | 0.2 | 0.7 | 8.1 (6.8, 9.3)c | <0.5 | 0.2 | <0.3 | NDd |
| 55 (adult) | 0.1 | 48.8 | 26.4 ± 2.1 | 4.1 | 10.0 | 46.0 | 75 |
| 60 (adult) | 0.8 | 55.8 | 25.0 ± 2.3 | 9.6 | 17.9 | 72.0 | 39 |
| Poolsa | |||||||
| 1 (9.5) | 1.2 | 114 | 16.0 (14.0, 18.0)c | 3.2 | 10.0 | 107 | 73 |
| 2 (4.5) | <0.1 | 13.9 | 20.3 ± 1.8c | 0.2 | 2.4 | 16.6 | 54 |
| 3 (4.6) | ND | 12.0 | 13.0 ± 0.8e,f | 0.8 | 3.5 | 13.5 | 73 |
| 4 (2.6) | <0.2 | 2.8 | 7.0 ± 0.9f | <0.5 | 0.3 | 5.4 | 89 |
Equal volumes of sera from each individual in groups 1 to 4 described in Table 1 were pooled to make pools 1 to 4, respectively.
In an ELISA in the presence of soluble OAc-negative group C polysacharide (10 to 25 μg/ml). The solid-phase antigen was adipic-dihydrazide-derivatized OAc-positive group C polysaccharide, which is known to express both OAc-positive and OAc-negative epitopes (see text).
SE not calculated since only two independent measurements were performed. The values from each determination are given in parentheses.
ND, not done because of insufficient concentrations of anticapsular antibody.
P < 0.02 (for comparison of mean avidities of pools 2 and 3).
P < 0.01 (for comparison of mean avidities of pools 3 and 4).
With one exception, the antibody concentrations measured in the serum pools were similar to the respective geometric mean antibody concentrations of the individual sera that made up the pools. The exception was the postvaccination serum pool from children immunized at 8 to 9 years of age (postimmunization pool 1), which had total and IgG anticapsular antibody concentrations of 114 and 107 μg/ml, respectively. The corresponding geometric means of the antibody concentrations of this group were lower, 24.5 and 16.9 μg/ml. These discrepant results were reproducible upon repeated assays. Each of the serum pools was prepared from equal volumes of sera from children in groups 1 to 4, respectively (Table 1). Sera from two individuals in group 1 had antibody concentrations more than 10-fold higher than the geometric mean antibody concentration of the group. Inclusion of these two outlier sera in the pool may have accounted for the discrepancy between the antibody concentration measured in the pool and the respective geometric mean antibody concentration of the group.
All of the children and adults in this study were immunized with vaccine prepared from OAc-positive group C polysaccharide. Nevertheless, with the exception of subject 60, 50% or more of the IgG group C anticapsular antibody binding in the ELISA was inhibited by soluble OAc-negative polysaccharide (Fig. 1 and Table 3). Previous work by Arakere and Frasch also demonstrated that a substantial amount of the serum anticapsular antibody in individuals immunized with a meningococcal polysaccharide vaccine was directed towards epitopes on the OAc-negative group C polysaccharide (3).
Table 4 summarizes the bactericidal antibody titers of the pre- and postimmunization serum pools and the corresponding titers of the three representative pre- and postimmunization sera from the adults. When tested against either group C strain 4243 (OAc-positive) or 4335 (OAc-negative), all preimmunization sera had bactericidal titers of <1:4 The postimmunization serum from subject 54, an adult known to be a low anticapsular antibody responder (Table 3), also lacked bactericidal activity. The postimmunization sera from the two high-responder adults (subjects 55 and 60), and the postimmunization serum pool from the children immunized at 8 to 9 years of age, had high titers of bactericidal activity against both group C test strains (Table 4). The postimmunization serum antibody concentrations required for 50% killing of the OAc-positive strain 4243 (BC50) ranged from 1.1 to 2.3 μg/ml, which were approximately 3- to 10-fold higher than the respective concentrations required for killing of the OAc-negative strain 4335 (Table 4). Note, for calculating the BC50 concentrations against the OAc-negative strains, we only considered the fraction of the serum anticapsular antibody concentrations that was specific for OAc-negative epitopes (i.e., inhibited in the ELISA by soluble OAc-negative polysaccharide).
TABLE 4.
Serum bactericidal antibody responses of selected adults and children to meningococcal vaccinationa
| Subject or pool no. (mean age [yr]) | Strain 4243 (OAc positive)
|
Strain 4335 (OAc negative)
|
||||
|---|---|---|---|---|---|---|
| Reciprocal titer in preimmunization sera | Postimmunization sera
|
Reciprocal titer of preimmunization serab | Postimmunization sera
|
|||
| 1/Titerb | BC50c | 1/Titer | BC50c | |||
| Subjects | ||||||
| 54 (adult) | <4 | <4 | INDETd | <4 | <4 | INDET |
| 55 (adult) | <4 | 21 | 2.3 | <4 | 101 | 0.4 |
| 60 (adult) | <4 | 50 | 1.1 | <4 | 170 | 0.1 |
| Pools | ||||||
| 1 (9.5) | <4 | 100 | 1.1 | <4 | 300 | 0.3 |
| 2 (4.5) | <4 | <4 | >3.5 | <4 | 12 | 0.6 |
| 3 (4.6) | NDe | <4 | >3.0 | ND | <4 | >2.2 |
| 4 (2.6) | <4 | <4 | INDET | <4 | <4 | INDET |
For description of pools, see footnote a of Table 3.
Dilution of sera giving 50% killing in the presence of human complement. Note that all sera with titers of <1:4 were also negative (titer, <1:4) when retested with rat complement.
BC50 for strain 4243 was calculated from the total antibody concentration measured by the RABA divided by the reciprocal bactericidal titer. A similar calculation was done to calculate the BC50 of strain 4335 except that the total anticapsular antibody concentration was adjusted to take into consideration the percentage inhibition by soluble OAc-negative polysaccharide (i.e., the portion specific for OAc-negative polysaccharide equals the total antibody concentration measured by RABA multiplied by the percent inhibited by OAc-negative polysaccharide in the ELISA (see Fig. 1).
INDET, indeterminate because of low concentrations of anticapsular antibody.
ND, not done.
The three postimmunization serum pools from children immunized before 5 years of age had no detectable bactericidal activity when tested against the OAc-positive strain 4243 (Table 4), and only pool 2, from the 4-year-olds immunized in Canada, was bactericidal for OAc-negative strain 4335 (titer = 1:12). The respective concentrations of anticapsular antibody required for 50% bacteriolysis in the postimmunization serum pools from children immunized before 5 years of age were two- to sevenfold higher than those for the serum pool from 8- to 9-year-olds, or for the individual sera from the two control adult responders.
Antibody avidity.
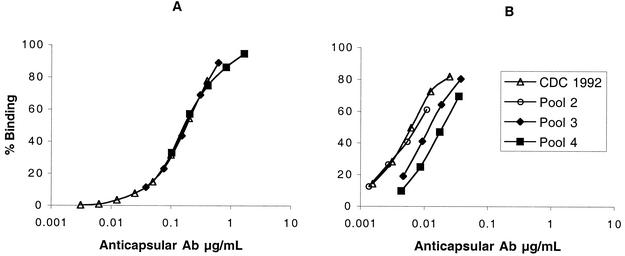
One possible explanation for low bactericidal activity of the serum pools from the children is low antibody avidity. Figure 2 shows the ability of different concentrations of anticapsular antibodies in postimmunization serum pools 3 (mean age of children, 4.6 years) and 4 (mean age of children, 2.6 years) to bind to radiolabeled group C polysaccharide. For comparison is shown the corresponding binding of group C antibodies in the reference CDC1992 pool prepared from sera of adults given meningococcal polysaccharide vaccine (27). When a high radioantigen concentration is used in the assay (Fig. 2A), which detects both low- and high-avidity antibodies, the respective binding curves of the three pools overlap each other. In a separate experiment not shown, there was similar overlap of the respective binding curves of the CDC reference pool and pools 1 and 2 from the immunized children from Canada (mean ages of 9.5 and 4.5 years, respectively). In contrast, when a 25-fold-lower concentration of radioantigen was used in the RABA (Fig. 2B), conditions which favor detection of high-avidity antibodies (26), it took approximately four- to fivefold-higher concentrations of anticapsular antibody from pool 4 to give equivalent binding to that of antibodies in the reference CDC pool. With the low-antigen assay, binding of pool 3 was intermediate between that of pool 4 and that of the CDC1992 pool, and binding of pool 2 was indistinguishable from that of the CDC pool (Fig. 2B). The Ka ± SE calculated from these and other RABA data (not shown) were 7 ± 0.9 nM−1, 13 ± 0.8 nM−1, 20 ± 2 nM−1, 16 nM−1 (SE not calculated because the sample was assayed only twice, with values of 14 and 18 nM−1), and 17 ± 2 nM−1, for pools 4, 3, 2, and 1 (Table 3) and the reference CDC1992 serum pool, respectively.
FIG. 2.
Binding of anticapsular antibodies (Ab) to radiolabeled group C polysaccharide. (A) When a high antigen dose is used both high- and low-avidity antibodies are detected. (B) When a low antigen dose is used, detection of higher-avidity antibodies is favored. It takes four- to fivefold-higher concentrations of anticapsular antibody from pool 4, a low-avidity pool, to give equivalent binding to that of antibodies in the CDC1992 reference pool. The dose-response binding of pool 3 in the low-antigen assay is between that of pool 4 and that of the CDC1992 reference pool, and pool 3 is calculated to have an intermediate avidity Ka between that of pool 4 and that of the CDC pool (Table 3).
Passive protective activity in infant rats.
Although the presence of serum bactericidal antibody correlates with protection against developing meningococcal disease, absence of bactericidal activity does not necessarily imply susceptibility to disease. To investigate this question in the pools of serum from the children immunized in this study, we adapted the infant-rat challenge model previously used for group B meningococci (40) to investigate passive protection against group C bacteremia. As negative controls in these experiments, a total of 41 infant rats were pretreated i.p. at time zero with 100 μl of 1:2 or 1:5 dilutions of sera obtained before immunization from four adults. Of the 41 animals, 23 were challenged i.p. with strain 4243 (OAc positive), and 18 were challenged i.p. with strain 4335 (OAc negative). For both strains the challenge bacterial dose used was between 500 and 1,000 CFU/rat. All 41 animals pretreated with preimmunization sera had bacteremia in blood obtained 18 h after the bacterial challenge (typically, 300,000 to >500,000 CFU per ml of blood).
Table 5 summarizes the results of pretreatment of rats with pre- or postimmunization sera from the three representative adults on the development of bacteremia 18 h after challenge with group C strain 4243 (experiment 1A) or 4335 (experiment 1B). None of the rats treated with 0.04 μg of antibody in postimmunization serum from subject 54, the control low responder adult with a bactericidal titer of <1:4, was protected against challenge by either strain (Table 5). However, the same dose of antibody in postimmunization sera from adult subjects 55 or 60 was highly protective against strain 4243, and a dose of 0.008 μg per rat gave partial protection against this strain. These data imply that there are important qualitative differences in anticapsular antibodies that may influence protective activity.
TABLE 5.
Passive protection of infant rats challenged with group C N. meningitidis with adult sera
| Subject no. and serumb | Dilution | Strain 4243 (OAc positive) (expt 1A)a
|
Strain 4335 (OAc negative) (expt 1B)a
|
||||
|---|---|---|---|---|---|---|---|
| Antibody dose (μg/rat)c | No. of animals positive for bacteremia/total no. tested | CFU/ml (geometric mean [103]) | Antibody dose (μg/rat) | No. of animals positive for bacteremia/total no. tested | CFU/ml (geometric mean [103]) | ||
| 54 | |||||||
| Pre | 1:2 | 0.01 | 5/5 | >500 | ≤0.01 | 5/5 | 313 |
| Post | 1:2 | 0.04 | 5/5 | >500 | ≤0.04 | 5/5 | >500 |
| 55 | |||||||
| Pre | 1:5 | 0.002 | 6/6 | >500 | ≤0.002 | 3/3 | >500 |
| Postd | 1:20 | 0.2 | 0/6 | <0.001 | 0.14 | 3/5 | 0.897 |
| Post | 1:100 | 0.04 | 0/6 | <0.001 | 0.03 | 5/6 | 21 |
| Post | 1:500 | 0.008 | 3/6 | 0.49 | 0.006 | 5/6 | 67 |
| 60 | |||||||
| Pre | 1:5 | 0.02 | 6/6 | >500 | ≤0.02 | 5/5 | 378 |
| Post | 1:28 | 0.2 | 0/7 | <0.001 | 0.08 | 1/5 | 0.008 |
| Post | 1:140 | 0.04 | 1/7 | 0.004 | 0.02 | 5/6 | 3 |
| Post | 1:700 | 0.008 | 6/7 | 6.0 | 0.003 | 6/6 | >500 |
Experiments 1A and 1B were done on separate days. Results of experiments 2A, 2B, 3A, and 3B are shown in Table 6.
Pre, preimmunization; post, postimmunization.
The same respective dilutions of sera from each subject were tested against both strains. The antibody dose per rat for strain 4243 was calculated from the total antibody concentration measured by the RABA divided by the dilution. The antibody dose per rat for the OAc-negative strain 4335 was adjusted to take into consideration the percentage of antibody inhibited by soluble OAc-negative polysaccharide (see Fig. 1 and Table 4, footnote c).
For subject 55, serum from 1 month postimmunization (39.5 μg/ml) was used for the passive-protection experiment with strain 4243. For the passive protection experiment with strain 4335, the serum from 2 months postimmunization was used because of insufficient quantity of the sample from 1 month postimmunization. Because the 2-month sample had a slightly higher antibody concentration than the 1-month sample (48.8 μg/ml), the 2-month serum was diluted 1:26, 1:130, and 1:650 to achieve doses of 0.2, 0.04, and 0.008 μg of total anticapsular antibody/rat, respectively.
Table 6 shows the result of passive-protection experiments performed with serum pools from immunized children. Pool 1, which was prepared from serum samples of children immunized at a mean age of 9.5 years and which was highly bactericidal in vitro, was not tested for protection in the animal model since the purpose of these experiments was to determine whether sera from immunized younger children that lacked bactericidal activity could confer protection. Experiment 2A compared protection conferred by postimmunization serum pools obtained from Amish children immunized at mean ages of 2.6 (pool 4) and 4.6 (pool 3) years against challenge with strain 4243. Experiment 2B tested the same serum pools in rats challenged with strain 4335. Experiment 3A compared protection conferred by postimmunization pools obtained from two groups of children immunized at mean ages of 4.6 years (pool 3, U.S. Amish children) and 4.5 years (pool 2, Canadian children) against challenge with strain 4243. Experiment 3B tested the same serum pools in rats challenged with strain 4335. In experiments 2A and 3A, all 24 rats pretreated with preimmunization sera from the control adult 55 or pools made from preimmunization sera of children or with PBS had bacteremia following challenge with strain 4243 (>400,000 CFU/ml 18 h after challenge). Similarly, all 24 animals given these preimmunization sera and challenged with strain 4335 in experiments 2B and 3B developed bacteremia (geometric mean, >500,000 CFU/ml at 18 h after challenge). In both experiments 2A and 3A, a dose per rat of 0.2 μg of postimmunization serum from the adult positive control completely protected 12 of 12 rats challenged with strain 4243, and in experiments 2B and 3B, a dose per rat of 0.14 μg of the positive adult control serum completely protected 11 of 12 rats challenged with strain 4335. The respective results with the positive and negative control sera in these experiments were similar to those of experiment 1A and 1B (compare Table 6 and Table 5).
TABLE 6.
Passive protection of infant rats with pediatric serum poolsa
| Expt no. | Subject or pool no. (age [yr]) and serum sampleb | Dilution | Strain 4243 (OAc positive) (expt A)
|
Strain 4335 (OAc negative) (expt B)
|
||||
|---|---|---|---|---|---|---|---|---|
| Antibody dose (μg/rat)c | No. of animals positive for bacteremia/ total no. tested | CFU/ml (geometric mean [103]) | Ab Antibody dose (μg/rat) | No. of animals positive for bacteremia/ total no. tested | CFU/ml (geometric mean [103]) | |||
| 2 | Subject 55 (adult) | |||||||
| Pre | 1:5 | 0.002 | 7/7 | 434 | ≤0.002 | 7/7d | >500 | |
| Post | 1:26 | 0.2 | 0/7 | <0.001 | 0.14 | 0/7 | <0.001 | |
| Post | 1:130 | 0.04 | 0/7 | <0.001 | 0.03 | 3/7d | 0.05 | |
| Post | 1:650 | 0.008 | 5/7 | 1.69 | 0.006 | 7/7 | >500 | |
| Pool 4 (2.6) | ||||||||
| Pre | 1:2 | <0.005 | 7/7 | >500 | ≤0.005 | 7/7 | >500f | |
| Post | 1:1.4 | 0.2 | 5/5 | >500 | 0.18 | 3/3 | 49f | |
| Post | 1:5 | 0.04 | 7/7d | >500 | 0.05 | 7/7 | >500 | |
| Pool 3 (4.6) | ||||||||
| Post | 1:2 | 0.6 | 0/5 | <0.001 | NDe | ND | ND | |
| Post | 1:6 | 0.2 | 0/7 | <0.001 | 0.15 | 3/7c | 0.101 | |
| Post | 1:30 | 0.04 | 3/7 | 0.022 | 0.03 | 7/7 | 314 | |
| 3 | Negative control | |||||||
| PBS | 5/5 | >500 | 5/5 | >500g | ||||
| Subject 55 (adult) | ||||||||
| Post | 1:26 | 0.2 | 0/5 | <0.001 | 0.14 | 1/5 | 0.002 | |
| Post | 1:130 | 0.04 | 3/3 | 7.4 | 0.03 | 5/5 | 108 | |
| Pool 3 (4.6) | ||||||||
| Post | 1:6 | 0.2 | 4/5 | 0.04h | 0.15 | 5/5 | 23g | |
| Post | 1:30 | 0.04 | 5/5 | 197 | 0.03 | 5/5 | >500 | |
| Pool 2 (4.5) | ||||||||
| Pre | 1:5 | <0.02 | 5/5 | >500 | ≤0.02 | 5/5 | >500g | |
| Post | 1:6 | 0.2 | 0/4 | <0.001h | 0.13 | 2/5 | 0.045 | |
| Post | 1:30 | 0.04 | 5/5 | 76 | 0.03 | 5/5 | >500 | |
Experiments 2A and 2B and experiments 3A and 3B were done on separate days. For description of pools, see footnote a of Table 3.
Pre, preimmunization; post, postimmunization.
Antibody doses were calculated as described in footnotes b and c to Table 5.
One rat in each of these groups was dead 18 h after bacterial challenge. These animals were presumed to be positive for bacteremia. For calculation of geometric mean CFU per milliliter, the dead animals were assigned values of 1,000,000 CFU/ml of blood.
ND, not done.
P > 0.10 (for comparison of geometric mean CFU per milliliter after treatment with postimmunization serum pool 4 to that of animals treated with preimmunization serum).
P = 0.05 (for comparison of geometric mean CFU per milliliter after treatment with postimmunization serum pool 3 to that of the combined group of 10 animals treated with either preimmunization serum or PBS).
P < 0.03 (for comparison of geometric mean CFU per milliliter after treatment with antibody (0.2 μg/rat) from postimmunization serum pool 3 to that of animals treated with antibody (0.2 μg/rat) from postimmunization serum pool 2).
In experiment 2A, doses per rat of 0.2 and 0.04 μg of anticapsular antibody in postimmunization serum pool 3, obtained from Amish children immunized at an average age of 4.6 years, were completely or partially protective (>99% decrease in the number of CFU per milliliter of blood) against strain 4243. In contrast, comparable doses of antibody in pool 4 from Amish children immunized at a mean age of 2.6 years gave no protection against this strain (Table 6). A similar trend was observed in animals pretreated with antibody in experiment 2B and challenged with strain 4335 (partial protection with a dose per rat of 0.15 μg of serum anticapsular antibody from the 4.6-year-olds but no significant protection at a dose per rat of 0.18 μg of serum antibody from the 2.7-year-olds. The postimmunization control serum antibody from adult 55 completely or partially protected against bacteremia caused by either strain at a dose of 0.03 to 0.04 μg per rat. Thus, there was an inverse correlation between age of immunization and dose of serum anticapsular antibody required to confer protection in this model. Also, some sera that lack bactericidal activity can be highly protective in vivo. For example, postimmunization serum pools 2 and 3 had no detectable bactericidal activity against strain 4243 (Table 4) but conferred protection against this strain in the animal model (Table 6). Postimmunization serum pools 2 and 3 also had no detectable bactericidal activity when tested with infant or adult rat serum as sources of complement; thus, passive protection by these serum pools may have resulted from a mechanism other than bactericidal activity such as opsonization.
In experiments 3A and 3B (Table 6), doses per rat of 0.13 to 0.2 μg of anticapsular antibody in postimmunization serum pool 2 from Canadian children immunized at an average age of 4.5 years were completely protective against strain 4243 and partially protective against strain 4335. This serum pool lacked bactericidal activity against strain 4243 and had a titer of antibody against strain 4335 of 1:12 (Table 3). Similar doses of antibody from pool 3, obtained from Amish children with a mean age of 4.6 years, were partially protective against strain 4243 but in this experiment gave minimal protection against strain 4335. Similar doses per rat of antibody from serum of the control immunized adult 55 completely protected against strain 4243 and partially protected against strain 4335.
Relationship between antibody avidity and passive protective activity in infant rats.
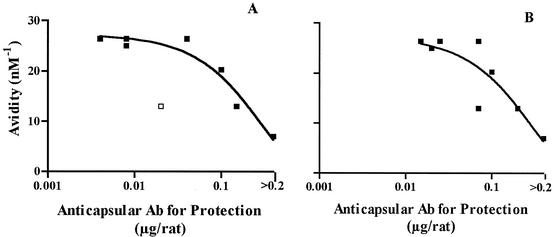
Fig. 3 shows the relationship between the Ka calculated for each postimmunization control serum or serum pool tested for passive protection and the protective dose of anticapsular antibody determined for each of the passive-protection experiments. The protective antibody dose was defined as the dose per rat that gave a 2-log10 decrease in the number of CFU per milliliter, compared to that of control animals pretreated with preimmunization serum or PBS. Data from one sample (adult 54 with a Ka of 8.1 nM−1) were excluded from the analyses since the highest antibody dose tested for protection of that serum was 0.04 μg/rat (Table 5) and, thus, the protective dose could not be defined. For both challenge strains, the higher the avidity of the serum anticapsular antibody, the lower the antibody dose required for conferring protective activity. The r2 value calculated for the OAc-positive test strain 4243 (Fig. 3A) was 0.60 (P < 0.03), and that for the OAc-negative test strain (panel B) was 0.69 (P < 0.01). Excluding the one outlier (see legend to Fig. 3A), the r2 value calculated for the OAc-positive test strain 4243 was 0.92 (P < 0.001).
FIG. 3.
Relationship between antibody (Ab) avidity and dose of antibody per rat required for passive protective activity (protective activity was defined as a 2-log10 decrease in the number of CFU per milliliter, compared to that of control animals pretreated with preimmunization serum or PBS). (A) Challenge by group C OAc-positive strain 4243. The point shown as an open square was considered an outlier and was excluded for purposes of the curve fit. (B) Challenge by group C OAc-negative strain 4335. The respective r2 values calculated by analysis of variance nonlinear regression were 0.60 for strain 4342 (P < 0.03) and 0.69 for strain 4335 (P < 0.01). Excluding the outlier for strain 4243, the r2 value was 0.92 (P < 0.001).
DISCUSSION
The relationship between serum group C anticapsular antibody concentrations and protection from developing meningococcal disease is unknown. Young children immunized with meningococcal polysaccharide vaccine are reported to develop high serum group C anticapsular antibody responses as measured by ELISA (10, 37) or RABA (15, 16). Typically, however, there is little or no detectable bactericidal activity in this age group (10, 29, 37), particularly if measured with human complement (34). Our serologic results are consistent with these earlier observations. Children immunized at 2 to 4 years of age showed 20- to 80-fold increases in anticapsular antibody concentrations as measured by the RABA, comparing the respective geometric mean concentrations measured in sera obtained 1 to 2 months after vaccination to those in preimmunization sera (Table 1). However, the complement-mediated bactericidal antibody responses were poor (Tables 2 and 4), particularly when measured against the OAc-positive strain 4243; only 13% of the 4-year-olds developed titers of antibody to this strain of 1:4 or more after vaccination, a threshold predictive of protection against developing disease (6, 19). In experiments not shown, we also measured bactericidal antibody titers of sera from 22 immunized 4-year-old Canadians, including the 15 children in group 2, using two other OAc-positive strains, C11 (also called 60E) (19) and RM1090, and two other OAc-negative strains, 89I and 1088, as the target organisms. We obtained similar respective results as those shown for strains 4243 and 4335. Strain C11 is a group C strain used by many laboratories for measurement of group C serum bactericidal activity (19, 36, 45). Thus, the low bactericidal titers measured with the two group C test strains used in the present study are likely to be relevant to titers measured against other group C target strains expressing the OAc-positive or OAc-negative capsular polysaccharide.
Considerable data support a relationship between the presence of serum bactericidal antibody and protection from developing invasive meningococcal disease (reviewed by Frasch [13]). However, not all persons whose sera lack bactericidal activity are necessarily susceptible to developing disease (19). To date, this inference has not been tested experimentally because of lack of a suitable animal model of group C meningococcal disease.
The infant-rat challenge model described herein for group C bacteremia is similar to that used previously by us for investigation of the protective activity of antibodies to group B organisms (40). For the group C model, we use a relatively low challenge dose of 500 to 5,000 CFU of either an OAc-positive or OAc-negative strain. The challenge dose is prepared from organisms grown to mid-log phase in broth culture, and the bacteria are separated by centrifugation, washed, resuspended in buffer, and given i.p. In unprotected animals, the density of bacteria in the bloodstream increases within 18 h to >500,000 CFU/ml. Pretreatment of the animals with antibody can completely prevent bacteremia. Thus, the model permits investigation of the protective activity of group C anticapsular antibodies with different fine antigenic specificities related to OAc in an in vivo setting where, in the absence of protective antibodies, the organism is rapidly replicating.
Our most important findings from this model are as follows. (i) Serum anticapsular antibodies that are elicited by meningococcal polysaccharide vaccination of children, and which lack complement-mediated bactericidal activity, can confer protection. (ii) In the absence of bactericidal activity, a high antibody avidity correlates with in vivo protective activity. (iii) Age of immunization affects antibody functional activity. The serum samples used in our studies were obtained in previous studies of different populations (i.e., Canadian children and U.S. Amish children) using different vaccines (tetravalent or bivalent) and different vaccine doses. As described below, we do not believe these potential confounders affected the principal results or conclusions of this study.
In the Canadian study, all subjects received the same dose of tetravalent vaccine, and their sera were stored under identical conditions (−70°C). Within each age group, selection of the subset of sera used in our study was random since only a small subset of the samples had been assayed previously. Although immunization of the children was performed in response to an outbreak of group C disease, rates of disease in the population were low (∼1/100,000 population [11, 29]). Also, colonization by group C strains is infrequent in the general population, even during outbreaks. Finally, the group C anticapsular antibody concentrations and bactericidal titers of the prevaccination sera of children in groups 1 and 2 were for the most part below detection (Tables 1 and 2), results consistent with lack of recent exposure to group C organisms. Thus, it is unlikely the antibody responses to vaccination of these subjects were affected by prior colonization by group C strains.
The Amish children in groups 3 and 4 received a bivalent meningococcal polysaccharide vaccine instead of the tetravalent vaccine, and one-fifth of the usual dose was given as part of a study of genetic factors affecting immune responses (24). However, both groups of Amish children were immunized in the same study, and the only difference between the two groups was the age of vaccination (mean age of 4.6 years for group 3 and mean age of 2.6 years for group 4). The serum pools from the two groups were compared directly in the same passive-protection experiments in infant rats (Table 6, experiment 2A, challenge by an OAc-positive strain, and experiment 2B, challenge by an OAc-negative strain). The results were unambiguous. Both serum pools lacked detectable complement-mediated bactericidal activity against both group C strains (Table 4). Nonetheless, the pool from the older children with higher antibody avidity (13 nM−1) protected the rats from bacteremia at doses of 0.2 or 0.04 μg/rat, whereas the serum pool with lower avidity (7.0 nM−1) did not confer protection at either dose. In the same experiment, the positive control serum from an immunized adult conferred protection at antibody doses of 0.2, 0.04, and 0.008 μg/rat. The superior protection of the adult serum correlated with the presence of complement-mediated bactericidal activity and also had higher-avidity antibody (26.4 nM−1) than that of the serum pools from the two groups of children.
In experiments 3A and 3B (Table 6), we compared protective activity of serum pools prepared from two groups of children immunized at mean ages of 4.5 years (group 2, Canadian) and 4.6 years (group 3, Amish). The respective geometric mean pre- and postimmunization anticapsular antibody concentrations of the two groups were similar (Table 1), as were the respective total and IgG anticapsular concentrations of the pools prepared from the sera from the two groups. Although in this experiment there are many potential confounders (i.e., different vaccine, dose, and genetic backgrounds), the data confirm that serum pools without bactericidal activity against strain 4243 (pools 2 and 3) can be highly protective in the model. Also, the adult positive control serum with the highest antibody avidity (Ka = 26.4 nM−1) gave the best protection, followed by the serum pool from group 2, which had a somewhat lower avidity (20.3 nM−1 [Canadian children]), with the lowest protection observed in animals pretreated with antibody from serum pool 3 from the Amish children, which had the lowest avidity (13 nM−1). Thus, the results of the different passive-protection experiments with the different serum pools were consistent with respect to our findings that pools that lack bactericidal activity can confer protection, the finding of a correlation between increasing antibody avidity and increasing protective activity, and the effect of older age of immunization and increased protective activity.
The RABA method used for measurement of antibody avidity in this study was adopted from the procedure originally described by Griswold et al. (26) in 1989 to measure the avidity of Hib anticapsular antibodies. In contrast to subsequently described ELISA methods that use chaotropic agents to dissociate antigen-antibody complexes (2, 17, 18) and give estimates of avidity (avidity index), the RABA method (Fig. 2) allows direct calculation of a Ka. Using this method, there was a good correlation between antibody avidity and the dose of serum anticapsular antibody required for protective activity (Fig. 3). Similar correlations between antibody avidity and anticapsular antibody functional activity have been observed for Hib (1, 33, 46) and Streptococcus pneumoniae (47, 49). Average affinity of the MAbs to phosphorylcholine also correlated with passive protective activity against experimental pneumococcal infection (41). However, in the absence of serum bactericidal activity against N. meningitidis, predicting protective antibody activity is still a complex undertaking. In addition to antibody concentration and avidity, the quality of the antibody can be affected by the isotype and specific epitopes recognized.
Our experimental data from the passive protection model are consistent with epidemiologic observations supporting protective efficacy of meningococcal vaccine when given to 4-year-old children (11, 44, 48), albeit less protective than in adults (11, 19, 20). The efficacy of group C polysaccharide vaccine in 2-year-old children is controversial, with some studies showing limited or no efficacy (11, 48). Although the experimental passive protection data reported herein for this age group are limited to sera obtained from only one group of Amish children immunized at 2 to 3 years of age, our results are consistent with the low vaccine efficacy in this age group (i.e., absence of both bactericidal and protective activity in pool 4).
Recently, meningococcal group C polysaccharide-protein conjugate vaccines were licensed in Europe and Canada (two prepared from OAc-positive polysaccharide and one prepared from OAc-negative polysaccharide). Following their introduction into routine and mass catch-up immunization in the United Kingdom, the number of cases of group C meningococcal disease has declined dramatically (28, 39, 43). The conjugated polysaccharide has T-cell-dependent antigenic properties. As such, these vaccines are more immunogenic in infants and young children than unconjugated group C polysaccharide, and the conjugate vaccines also elicit serum antibodies with greater avidity and bactericidal activity (6, 8, 10, 23, 32, 34, 35) and prime for memory antibody responses (7, 8, 35). In the future it will be of interest to investigate the protective activity of conjugate-vaccine-induced antibodies in the infant rat model and to dissect the relative protective role of antibodies elicited by OAc-positive or -negative polysaccharide.
Acknowledgments
This work was supported, in part, by grants RO1 AI 45642 and AI46464 from the National Institutes of Allergy and Infectious Disease, NIH, and a grant from Aventis Pasteur.
Leslie M. Cayco, Christian C. DaCosta, and Quandra T. McGrue, Children's Hospital Oakland Research Institute, provided expert technical assistance. We are grateful to Alexander Lucas for helpful discussions and to Noni MacDonald (Dalhousie University, Halifax, Canada), a co-investigator of the Canadian study, for providing the serum samples from groups 1 and 2.
Editor: D. L. Burns
REFERENCES
- 1.Amir, J., X. Liang, and D. M. Granoff. 1990. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr. Res. 27:358-364. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. [DOI] [PubMed] [Google Scholar]
- 3.Arakere, G., and C. E. Frasch. 1991. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect. Immun. 59:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artenstein, M. S., R. Gold, J. G. Zimmerly, F. A. Wyle, H. Schneider, and C. Harkins. 1970. Prevention of meningococcal disease by group C polysaccharide vaccine. N. Engl. J. Med. 282:417-420. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1975. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J. Biol. Chem. 250:1926-1932. [PubMed] [Google Scholar]
- 6.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, R., A. J. Fox, P. C. Richmond, S. Clark, F. Sadler, J. Findlow, R. Morris, N. T. Begg, and K. A. Cartwright. 2000. Induction of immunological memory in UK infants by a meningococcal A/C conjugate vaccine. Epidemiol. Infect. 124:427-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, R., D. Goldblatt, N. Andrews, P. Richmond, J. Southern, and E. Miller. 2001. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J. Infect. Dis. 184:377-380. [DOI] [PubMed] [Google Scholar]
- 9.Borrow, R., E. Longworth, S. J. Gray, and E. B. Kaczmarski. 2000. Prevalence of de-O-acetylated serogroup C meningococci before the introduction of meningococcal serogroup C conjugate vaccines in the United Kingdom. FEMS Immunol. Med. Microbiol. 28:189-191. [DOI] [PubMed] [Google Scholar]
- 10.Campagne, G., A. Garba, P. Fabre, A. Schuchat, R. Ryall, D. Boulanger, M. Bybel, G. Carlone, P. Briantais, B. Ivanoff, B. Xerri, and J. P. Chippaux. 2000. Safety and immunogenicity of three doses of a Neisseria meningitidis A + C diphtheria conjugate vaccine in infants from Niger. Pediatr. Infect. Dis. J. 19:144-150. [DOI] [PubMed] [Google Scholar]
- 11.De Wals, P., G. De Serres, and T. Niyonsenga. 2001. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Quebec. JAMA 285:177-181. [DOI] [PubMed] [Google Scholar]
- 12.Fijen, C. A., E. J. Kuijper, M. Drogari-Apiranthitou, Y. Van Leeuwen, M. R. Daha, and J. Dankert. 1998. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine. Clin. Exp. Immunol. 114:362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasch, C. E. 1995. Meningococcal vaccines: past, present and future, p. 245-283. In K. Cartwright (ed.), Meningococcal disease. John Wiley & Sons, New York, N.Y.
- 14.Garcia-Ojeda, P. A., M. E. Monser, L. J. Rubinstein, H. J. Jennings, and K. E. Stein. 2000. Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 68:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold, R., M. L. Lepow, I. Goldschneider, T. F. Draper, and E. C. Gotschlich. 1979. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J. Infect. Dis. 140:690-697. [DOI] [PubMed] [Google Scholar]
- 16.Gold, R., M. L. Lepow, I. Goldschneider, T. L. Draper, and E. C. Gotschlich. 1975. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Investig. 56:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldblatt, D., P. Richmond, E. Millard, C. Thornton, and E. Miller. 1999. The induction of immunologic memory after vaccination with Haemophilus influenzae type b conjugate and acellular pertussis-containing diphtheria, tetanus, and pertussis vaccine combination. J. Infect. Dis. 180:538-541. [DOI] [PubMed] [Google Scholar]
- 18.Goldblatt, D., A. R. Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112-1115. [DOI] [PubMed] [Google Scholar]
- 19.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotschlich, E. C., M. Rey, R. Triau, and K. J. Sparks. 1972. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J. Clin. Investig. 51:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granoff, D. M., R. K. Gupta, R. B. Belshe, and E. L. Anderson. 1998. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J. Infect. Dis. 178:870-874. [DOI] [PubMed] [Google Scholar]
- 23.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granoff, D. M., T. McKinney, E. G. Boies, N. P. Steele, J. Oldfather, J. P. Pandey, and B. K. Suarez. 1986. Haemophilus influenzae type b disease in an Amish population: studies of the effects of genetic factors, immunization, and rifampin prophylaxis on the course of an outbreak. Pediatrics 77:289-295. [PubMed] [Google Scholar]
- 25.Granoff, D. M., B. K. Suarez, J. P. Pandey, and P. G. Shackelford. 1988. Genes associated with the G2m(23) immunoglobulin allotype regulate the IgG subclass responses to Haemophilus influenzae type b polysaccharide vaccine. J. Infect. Dis. 157:1142-1149. [DOI] [PubMed] [Google Scholar]
- 26.Griswold, W. R., A. H. Lucas, J. F. Bastian, and G. Garcia. 1989. Functional affinity of antibody to the Haemophilus influenzae type b polysaccharide. J. Infect. Dis. 159:1083-1087. [DOI] [PubMed] [Google Scholar]
- 27.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jodar, L., I. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 29.King, W. J., N. E. MacDonald, G. Wells, J. Huang, U. Allen, F. Chan, W. Ferris, F. Diaz-Mitoma, and F. Ashton. 1996. Total and functional antibody response to a quadrivalent meningococcal polysaccharide vaccine among children. J. Pediatr 128:196-202. [DOI] [PubMed] [Google Scholar]
- 30.Lees, A., B. L. Nelson, and J. J. Mond. 1996. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine 14:190-198. [DOI] [PubMed] [Google Scholar]
- 31.Lemercinier, X., and C. Jones. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides from Neisseria meningitidis used in vaccine production. Carbohydr. Res. 296:83-96. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman, J. M., S. S. Chiu, V. K. Wong, S. Partidge, S. J. Chang, C. Y. Chiu, L. L. Gheesling, G. M. Carlone, and J. I. Ward. 1996. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA 275:1499-1503. [PubMed] [Google Scholar]
- 33.Lucas, A. H., and D. M. Granoff. 1995. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J. Immunol. 154:4195-4202. [PubMed] [Google Scholar]
- 34.MacDonald, N. E., S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff. 1998. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 280:1685-1689. [DOI] [PubMed] [Google Scholar]
- 35.MacLennan, J. M., F. Shackley, P. T. Heath, J. J. Deeks, C. Flamank, M. Herbert, H. Griffiths, E. Hatzmann, C. Goilav, and E. R. Moxon. 2000. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: a randomized controlled trial. JAMA 283:2795-2801. [DOI] [PubMed] [Google Scholar]
- 36.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, G. M. Carlone, et al. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maslanka, S. E., J. W. Tappero, B. D. Plikaytis, R. S. Brumberg, J. K. Dykes, L. L. Gheesling, K. B. Donaldson, A. Schuchat, J. Pullman, M. Jones, J. Bushmaker, and G. M. Carlone. 1998. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect. Immun. 66:2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michon, F., C. H. Huang, E. K. Farley, L. Hronowski, J. Di, and P. C. Fusco. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. (Basel) 103:151-160. [PubMed] [Google Scholar]
- 39.Miller, E., D. Salisbury, and M. Ramsay. 2001. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 20(Suppl. 1):S58-S67. [DOI] [PubMed] [Google Scholar]
- 40.Moe, G. R., P. Zuno-Mitchell, S. S. Lee, A. H. Lucas, and D. M. Granoff. 2001. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect. Immun. 69:3762-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoletti, C., X. Yang, and J. Cerny. 1993. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J. Immunol. 150:543-549. [PubMed] [Google Scholar]
- 42.Pastor, P., F. B. Medley, and T. V. Murphy. 2000. Meningococcal disease in Dallas County, Texas: results of a six-year population-based study. Pediatr. Infect. Dis. J. 19:324-328. [DOI] [PubMed] [Google Scholar]
- 43.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstein, N., O. Levine, J. P. Taylor, D. Evans, B. D. Plikaytis, J. D. Wenger, and B. A. Perkins. 1998. Efficacy of meningococcal vaccine and barriers to vaccination. JAMA 279:435-439. [DOI] [PubMed] [Google Scholar]
- 45.Santos, G. F., R. R. Deck, J. Donnelly, W. Blackwelder, and D. M. Granoff. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab. Immunol. 8:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 47.Sun, Y., Y. Hwang, and M. H. Nahm. 2001. Avidity, potency, and cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect. Immun. 69:336-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taunay, A. E., R. A. Feldman, C. O. Bastos, P. A. A. Galvao, J. S. Morais, and I. O. Castro. 1978. Avaliacao do efeito protector de vacina polissacaridica antimeningococica do groupo C, em criancas de 6 A 36 meses. Rev. Inst. Adolfo Lutz 38:77-82. [Google Scholar]
- 49.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]