Abstract
Bacterial ghosts are empty cell envelopes, which may be generated by the controlled expression of the PhiX174 lysis gene E in gram-negative bacteria to obtain vaccine candidates. We describe here the application of this technology to Helicobacter pylori. The lysis gene cassette was cloned into an Escherichia coli-Helicobacter pylori shuttle vector and introduced into an H. pylori recipient strain by bacterial conjugation. Temperature induction of the lysis gene cassette revealed a quantitative killing of the H. pylori culture without induction of lysis-resistant bacteria. Biochemical and transmission electron microscopic studies identified structurally intact H. pylori. Prophylactic oral vaccination experiments using these H. pylori ghosts in the BALB/c mouse model showed a significant reduction of the bacterial load in the ghost group, as measured by a quantitative bacterial reisolation procedure. Ten of 10 and 5 of 10 mice were protected, respectively, without the use of a mucosal adjuvant. Coadministration of ghosts with cholera toxin as mucosal adjuvant resulted in a complete protection of 10 of 10 and 8 of 8 mice against H. pylori challenge, with three animals showing a sterile immunity.
Helicobacter pylori, a prevalent gram-negative bacterium, infects half of the world's population, causing chronic active gastritis, which usually persists throughout life, unless the organism is eradicated (47). Although most infected individuals experience no symptoms, 15 to 20% develop peptic ulcer disease (41). Furthermore, chronic H. pylori infection confers a 3- to 12-fold increased risk of developing gastric malignancies, such as adenocarcinoma and low-grade B-cell lymphoma (16, 35, 43).
The use of vaccines for treatment and prevention of H. pylori infection has been explored as an alternative to standard multidrug regimens (10). The latter are known to induce antibiotic resistance in H. pylori strains (23) and cause the risk of reinfection following eradication (40). Animal studies have shown that immunization with H. pylori whole-cell sonicates or purified components is efficient for the prevention of infection, and, more importantly, for the treatment of preexisting infections (5, 8, 12, 14, 28, 31, 32, 50). All successful vaccination protocols included mucosal adjuvants, such as cholera toxin (CT) or Escherichia coli heat-labile toxin (LT), in addition to the antigen. Since CT and LT, and even the genetically detoxified forms of these adjuvants, induce diarrhea in humans (24, 33), it would be desirable to engineer a vaccine without the need of these adjuvants.
One attractive possibility includes the use of recombinant vaccine carrier strains that produce defined H. pylori vaccine antigens. Attenuated Salmonella vaccine strains (phoPc aroA) producing the H. pylori UreA and UreB subunits can induce protection in mice without the need of a mucosal adjuvant (6, 17). The purpose of the present study was to generate and test a certain form of inactivated bacteria, so-called “bacterial ghosts,” and test them for their potential to induce a prophylactic protection against a challenge with H. pylori in the well-established BALB/c mouse model.
Ghosts are empty bacterial cell envelopes without cytoplasm and DNA (46). They are generated by the tightly controlled expression of the cloned lysis gene E of bacteriophage PhiX174. The gene E-encoded protein was suggested to form a transmembrane tunnel in the bacterial cell wall, through which the cytoplasmic contents are expelled (46). Recent data by Bernhardt et al. (3) suggest that the lysis protein inhibits cell wall synthesis and thus kills the bacteria. Although the mechanism of genetic inactivation is still a matter of debate, the advantage of ghosts is that they share functional and antigenic determinants of the envelope with their living counterparts and thus represent ideal vaccine candidates.
Ghosts have been successfully generated in several gram-negative bacteria, such as Escherichia coli, Salmonella enterica serovar Typhimurium, Vibrio cholerae, Klebsiella pneumoniae, and Actinobacillus pleuropneumoniae (46). We demonstrate here for the first time the generation of inactivated H. pylori ghosts and show that they are able to protect mice against an oral challenge with an infectious dose of H. pylori.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori strains were grown on GC agar plates (Difco) supplemented with horse serum (8%), vancomycin (10 mg/liter), trimethoprim (5 mg/liter), and nystatin (1 mg/liter) (serum plates) and incubated for 24 to 48 h in a microaerophilic atmosphere (85% N2, 10% CO2, 5% O2) at 37°C. H. pylori strain P76 was originally obtained from H. Kleanthous, OraVax, Inc., and transformed to streptomycin resistance for optimal quantitative reisolation from the infected mouse stomach by streptomycin selection (250 mg/liter) (serum plates/strep). P79 is a derivative of H. pylori P1 transformed to streptomycin resistance with chromosomal DNA of a streptomycin-resistant H. pylori strain, NCTC 11637. E. coli strain DH5α (BRL) was grown on Luria-Bertani (LB) agar plates or in LB liquid medium (38) supplemented with chloramphenicol (30 mgl−1). Strain β2155 (9) was grown on the same medium supplemented with diaminopimelic acid (0.2 mM).
DNA manipulations.
Standard cloning and DNA analysis procedures were performed according to Sambrook et al. (38). Plasmid DNA was purified from E. coli by the boiling procedure, and E. coli cells for electroporation were prepared according to the protocol recommended for the Gene Pulser (Bio-Rad). Plasmid DNA was isolated from H. pylori strains by using Wizard minipreps (Promega) according to the protocol of the manufacturer.
Plasmid construction.
Plasmid pHPC38 is the product of subcloning a 2.4-kb DraI fragment of plasmid pAWC10 (unpublished data) into the unique BamHI restriction site of the E. coli-H. pylori shuttle vector pHel2, whereas the BamHI sticky ends of pHel2 have been made blunt by a Klenow fill-in reaction. Plasmid pAWC10, in analogy to plasmid pAWJ (22), carries the E-lysis cassette consisting of gene E, the lambda cI857 repressor gene, and the λPR promoter, which has been modified by a single-base-pair exchange to allow repression of the lethal gene E at temperatures up to 38°C.
Natural transformation and bacterial conjugation.
Shuttle and suicide plasmids were introduced into H. pylori strains by conjugation or natural transformation as described previously (15). H. pylori transformants or transconjugants carrying the shuttle plasmid pHPC38 were selected on serum plates containing 6 mg of chloramphenicol per liter.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (26) with a mini-slab apparatus. Proteins separated by SDS-PAGE were transferred to nitrocellulose membranes in a semidry blot apparatus at a current density of 0.8 mA/cm2. Unreacted sites of the nitrocellulose membrane were blocked with a 3% (wt/vol) solution of bovine serum albumin (BSA) in TBS (20 mM Tris-HCl [pH 7.5], 150 mM NaCl). The nitrocellulose membrane was then incubated with an appropriate dilution of antibody for 2 h and washed three times with TBS containing 0.5% (vol/vol) Tween 20. Subsequently, alkaline phosphatase conjugated to protein A was added to TBS containing 3% (wt/vol) BSA. After incubation for 1 h, the nitrocellulose membrane was washed three times with TBS containing 0.5% (vol/vol) Tween 20 and developed with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium.
Electron microscopic analysis.
For negative staining, thin carbon support films were prepared by indirect sublimation of carbon on to freshly cleaved mica. Samples were then absorbed to the carbon film and negatively stained with 1% (wt/vol) aqueous uranyl acetate (pH 4.5), as described previously (49). After air drying, samples were examined by transmission electron microscopy (TEM) in a Zeiss TEM 910 at an acceleration voltage of 80 kV. For embedding, after fixation of samples in 1% formaldehyde, samples were dehydrated in a graded series of acetone and embedded with Spurr epoxy resin according to the described protocol (42). Ultrathin sections were counterstained with uranyl acetate and lead citrate before examination in a Zeiss TEM 910.
Animals.
Age-matched (6 to 8 weeks) female BALB/c mice were obtained from RCC, Itingen, Switzerland. All protocols involving animal experimentation were approved by the Regierung von Oberbayern (Aktenzeichen 211-2531-60/98).
Growth of H. pylori for oral infection of mice.
For infection of mice, strain P76 was grown for 2 days on serum plates/strep at 37°C, harvested, and suspended in Brucella broth (Oxoid, Ltd., Basingstoke, England), and the final concentration was adjusted to 3.3 × 109 cells per ml. Mice were inoculated three times intragastrically at 2-day intervals with 0.3 ml of bacterial suspension (109 bacteria).
Preparation of H. pylori ghosts.
H. pylori P79(pHPC38) was grown on Brucella broth agar plates containing 10% horse serum and chloramphenicol (10 μg/ml). After a 2-day incubation at 36°C under a microaerobic atmosphere, cells were harvested in 10 ml of brain heart infusion (BHI) supplemented with 10% fetal calf serum and chloramphenicol (10 μg/ml) and grown for another 5 h under mild agitation (90 rpm). Before the culture reached the late-logarithmic growth phase, 10 to 50 ml of fresh medium was added. By repeating the addition of increasing amounts of fresh medium, the culture thus was expanded to 3.5 liters. When the growing culture reached an optical density at 600 nm (OD600) of 0.5, the incubation temperature was shifted up to 42°C to induce the gene E-mediated lysis process. After another 15 h of incubation at 42°C with monitoring of the OD550, the lysed culture was harvested. A centrifugation step (4°C, 10,000 × g, 10 min) served to pellet the ghosts, which were then resuspended in 100 ml of ice-cold phosphate-buffered saline (PBS). After the centrifugation and resuspension had been repeated twice more, the numbers of ghosts were determined under the microscope with a Thoma counting chamber, and aliquots of 2.5 × 109 ghosts per 100 μl were stored at −70°C until use.
Infection and reisolation of bacteria and quantitative culture.
At the endpoint of the experiment, mice were anesthetized by CO2 and sacrificed by cervical dislocation. The stomach was removed, weighed, and opened along the great curvature. For assessment of H. pylori colonization by quantitative culture, weighed stomachs were homogenized in 2 ml of Brucella broth by a hand homogenizer (Fisher Scientific, Germany), and serial dilutions of 1 in 20 were spread over the surface of serum plates/strep (performed in duplicate). The plates were incubated for 5 days, and colonies were counted to determine the number of CFU per gram of stomach tissue.
Statistical analysis.
In the vaccination experiments, statistical significance between the groups was determined by the nonparametric Wilcoxon signed-rank (two-tailed) test. P values of <0.05 are considered as significantly different.
RESULTS
Construction of a plasmid-based PhiX174 gene E lysis cassette for generation of H. pylori ghosts.
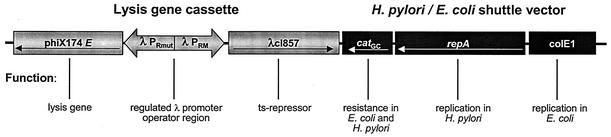
The E. coli-H. pylori shuttle plasmid pHel2 was used as the basis for the construction of an H. pylori lysis plasmid. This vector allows stable replication in both E. coli and H. pylori (20). The PhiX174 lysis gene cassette consists of the PhiX174 gene E, which is under the transcriptional control of the λPRmut promoter. λPRmut is a λPR promoter with a point mutation in the operator region leading to efficient repression by cI857 at higher temperatures than with the wild-type operator sequence. The promoter is repressed by binding of cI857 to the operator region at temperatures <38°C and induced at temperatures >38°C (22). The cI857 temperature-sensitive λ repressor is transcribed by the λPRM promoter in the opposite direction. (Fig. 1). The PhiX174 lysis gene cassette was ligated into the BamHI site of the multiple cloning site of shuttle plasmid pHel2 and transformed into E. coli DH5α. At temperatures below 38°C, recombinant plasmids could be isolated. One such plasmid, characterized by restriction analysis and designated as pHPC38, was used for all further experiments (Fig. 1).
FIG. 1.
Genetic map of pHPC38 carrying the PhiX174 gene E lysis cassette under control of the temperature-sensitive (ts) λ repressor binding to the λ promoter/operator region (left). The lysis cassette is carried by the pHel2 shuttle vector with replication functions for E. coli and H. pylori (right). catGC, chloramphenicol acetyltransferase gene.
Testing of the lysis gene cassette in E. coli by temperature induction.
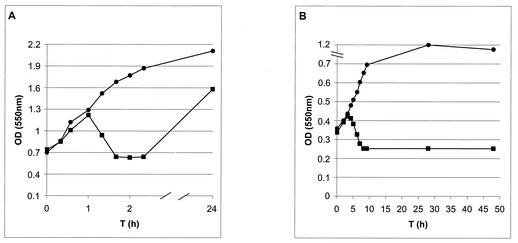
The functional expression of gene E from plasmid pHPC38 was tested in E. coli DH5α by growing the bacteria in LB medium and monitoring their growth by measuring the ODs of the culture at various time points (OD550). As a control, DH5α(pHel2) was used. The cultures were shifted from 28°C to 42°C at 0 h, which resulted in the functional inactivation of the cI857 repressor and transcription of E by the λPRmut promoter. After induction at 42°C, the OD of DH5α [pHPC38] increased to an OD550 of 1.25 within 1 h, but dropped rapidly to 0.6 (Fig. 2A). Strain DH5α carrying the cloning vector pHel2 alone showed a strong increase in its OD at the same time interval (OD550 = 1.9). DH5α(pHPC38) grown at the permissive temperature of <38°C showed growth characteristics similar to those of the control strain DH5α(pHel2) without lysis gene expression (data not shown). In E. coli, which is the natural host of bacteriophage PhiX174, mutant lysis-resistant bacteria had already appeared several hours after induction (data not shown). The experiment demonstrated both that the lysis gene cassette cloned in the E. coli-H. pylori shuttle vector was induced under the elevated temperature and that the system was functional in E. coli DH5α.
FIG. 2.
Growth and lysis curves of E. coli DH5α and H. pylori P79 harboring plasmid pHPC38 by temperature induction of gene E expression. (A) At time zero, the cultures were shifted from 28°C to 42°C. ▪, DH5α(pHPC38); •, DH5α(pHel2). Prolonged incubation at 42°C (up to 24 h) leads to a recovery of the culture DH5α(pHPC38) due to the emergence of lysis-resistant bacteria. (B) The H. pylori culture was grown at 35°C and shifted at time zero to 42°C. ▪, H. pylori P79(pHPC38); •, P79(pHel2). Prolonged incubation at 42°C (up to 48 h) does not lead to recovery of the culture P79(pHPC38).
Transfer of PhiX174 gene E into H. pylori by natural transformation or conjugation.
To test whether H. pylori was also sensitive to the PhiX174 gene E-mediated lysis procedure, we attempted to introduce the plasmid pHPC38 into H. pylori strains by natural transformation as well as by bacterial conjugation. Although different H. pylori strains were employed as recipients, natural transformation of the plasmid did not result in any transformants with plasmid pHPC38. With the E. coli host strain β2155 as a donor and H. pylori strain P79 as a recipient, bacterial conjugation resulted in H. pylori transconjugants after selection at 35°C. The plasmid was isolated from P79(pHPC38), and restriction digests revealed a stable replication of the plasmid at the permissive temperature (35°C) on serum plates.
To induce the temperature-dependent expression system, the recombinant strain P79[pHPC38] was grown in Brucella medium at 35°C to an OD550 of 0.35. The culture was then shifted to 42°C by gentle shaking (90 rpm). After temperature induction, the OD of the culture increased continuously for 3 h, a slight reduction in the OD550 was visible 4 h after induction, and the minimal OD was measured 9 h after induction (OD550 = 0.25) (Fig. 2B). Interestingly, the OD of the culture did not increase any further, even after prolonged incubation of up to 48 h (Fig. 2B). In contrast, H. pylori P79(pHel2) carrying the empty cloning vector was not hampered in its growth behavior at 42°C for up to 28 h and showed only a slight reduction after 48 h (Fig. 2B).
Analysis of the viability of the induced cultures by plating on serum plates revealed colony formation and growth of the P79(pHel2) control strain at the elevated temperature after 48 h, but no growth of P79(pHPC38) was observed (data not shown), indicating that no lysis-resistant H. pylori mutants arose and that the H. pylori culture was quantitatively inactivated. The generation of nonculturable coccoid forms of P79(pHPC38) following temperature induction could be excluded by phase-contrast microscopic inspection of the culture. In the P79(pHel2) control, coccoid forms were identified that probably resulted in the reduction of the OD550 of the culture and a slight reduction in the CFU after 48 h at 42°C. The fluorescence in situ hybridization (FISH) technique, developed recently in our laboratory, detects both vegetative and coccoid forms of H. pylori very efficiently (48), but interestingly, FISH did not detect protein E-inactivated H. pylori P79, indicating that rRNA was not accessible by FISH or was absent in the inactivated bacteria (data not shown).
Characterization of the inactivated H. pylori ghosts by TEM.
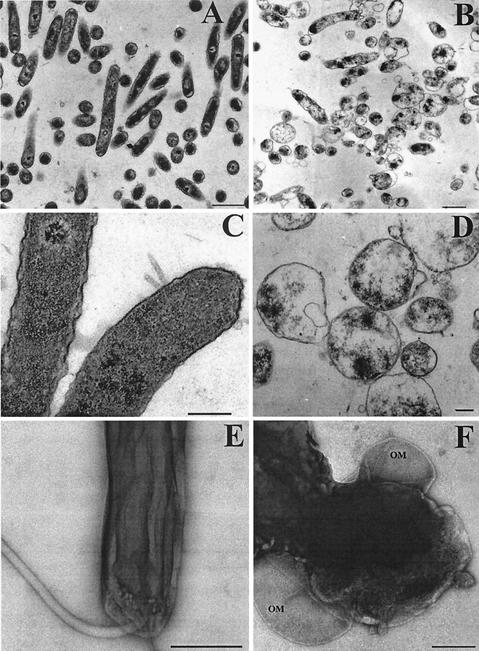
Next, TEM was used to compare P79 and P79(pHPC38), both grown at 42°C for 48 h (Fig. 3). By viewing ultrathin sections (Fig. 3A and C) or negative-stained specimens (Fig. 3E), H. pylori P79 showed no deviation from its vegetative form, and membranes appeared to be intact, indicating that the temperature stress did not have a deleterious effect on the H. pylori strain. The situation was completely different for P79(pHPC38). Analysis of ultrathin sections revealed a partial disintegration of the vegetative form, probably due to the strong disruption of the cytoplasmic membrane and the cell wall (Fig. 3B and D). The outer membrane looked mostly intact, but was sometimes found to be detached from the bacterial cytoskeleton (Fig. 3F, negative stain). The cytoplasmic content apparently did not leak out completely.
FIG. 3.
Characterization of PhiX174 protein E-inactivated H. pylori by TEM. P79 (A, C, and E) and P79(pHPC38) (B, D, and F) were grown for 48 h at 42°C in Brucella/FCS. After washing and fixation of bacteria, ultrathin sections were prepared (A to D), and negative staining (E and F) was performed. Ghosts (B, D, and F) show loss of cytoplasmic material and structural integrity. OM, outer membrane. Bars, 1 μm (A and B), 0.25 μm (C and D), and 0.5 μm (E and F).
To obtain further data on the protein level of P79(pHPC38), the genetically inactivated H. pylori cells were analyzed by Western blotting with specific antisera directed against proteins of the outer membrane and the cytoplasm (Table 1). Proteins such as RecA (39), UreA and UreB (25), and Pfr (2) could be identified in the lysates of the inactivated bacteria with the corresponding specific antisera. A significant reduction of these cytoplasmic proteins compared to the level of typical outer membrane proteins such as AlpA (37) or BabA (21) was not observed, although the Western blot procedure did not provide quantitative data.
TABLE 1.
Detection of protein antigens in H. pylori ghosts
| Antigen | Size (kDa) | Antiserum | Reference |
|---|---|---|---|
| AlpA | 53 | 214 | 37 |
| CagA | 129.7 | 257 | 36 |
| FlaA | 53.3 | 183 | 18 |
| Pfr | 19.3 | 198 | 2 |
| RecA | 37.6 | 263 | 39 |
| UreB | 62 | 201 | 17 |
Taken together, our data indicated that H. pylori P79 was efficiently inactivated by the PhiX-mediated lysis gene expression, but the cytoplasmic content of the bacteria was not completely expelled, as described for other ghosts induced by the PhiX-mediated expression system (46).
Evaluation of ghosts for prophylactic vaccination in the H. pylori mouse model.
Groups of 10 BALB/c mice were orally immunized with three doses of 2.5 × 109 ghosts or ghosts plus CT (10 μg per mouse) as mucosal adjuvant on days 0, 7, and 14. Control mice received PBS (infected group) or no treatment at all (naive group). Three weeks after the last immunization, all groups of mice except the naive group were challenged with a streptomycin-resistant, mouse-adapted H. pylori strain, P76 (109 CFU, three times with 2-day intervals), and 4 weeks later, the animals were sacrificed and gastric colonization was assessed by quantitative culture of P76 on serum plates containing streptomycin.
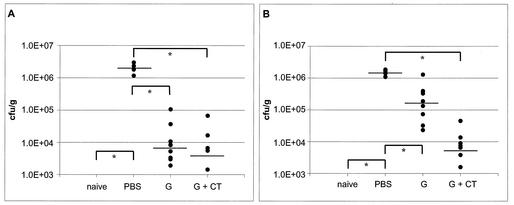
As compared to the naive group (Fig. 4A), the infection control (PBS group, Fig. 4A) shows significant infection, with a median of 1.85 × 106 CFU of P76 per g of stomach tissue. The ghost group (Fig. 4A) with a median of 6.75 × 103 is significantly reduced, compared to that in the infection control (∼96%). Only a slightly stronger reduction in colonization was found with the ghosts used together with CT as adjuvant (Fig. 4A), which resulted in a value of 3.78 × 103 as the median of infection (∼98%). The reduction in CFU over 2 logs indicates a protection of all animals in the ghost group, as well as the ghost plus CT group.
FIG. 4.
Prophylactic immunization with H. pylori ghosts in two independent experiments. (A) Ghost vaccination I. (B) Ghost vaccination II. Mice were immunized three times at weekly intervals and infected 3 weeks later. Bacterial colonization was assessed by quantitative reisolation of the challenge strain from the stomach by streptomycin selection on serum plates. Values were measured in CFU per gram of stomach tissue. naive, not infected; PBS, sham-immunized infection control; G, ghosts. Bars indicate the median of each of the groups. In ghost vaccination I, no bacteria were isolated from naïve (n = 5) animals. For the PBS group (n = 5), the median is 1.85 × 106 CFU/g. For ghosts (n = 10), the median is 6.75 × 103 CFU/g. For ghosts plus CT (n = 8 [two animals died]), the median is 3.78 × 103 CFU/g. In two mice, no H. pylori cells could be detected. The results for ghost vaccination II are as follows. For the PBS group (n = 9), the median is 1.44 × 106 CFU/g. For ghosts (n = 10), the median is 1.6 × 105 CFU/g. In one mouse, no H. pylori could be detected. For ghosts plus CT (10 mice), the median is 5.13 × 103 CFU/g. Two mice had an infection level of <103 CFU/g, and in one mouse, no H. pylori could be detected. *, P < 0.05.
In an attempt to reproduce and validate the data from the first experiment, a second experiment with a novel batch of bacterial ghosts was performed with the same vaccination strategy. Again, the animals vaccinated with ghosts plus CT showed the highest reduction rate in bacterial load (Fig. 4B), followed by the ghost group (Fig. 4B). The total reduction rate in the ghost group was less efficient than that in the first experiment, but was still significant. Five of 10 mice had enough of a reduction in the amount of CFU per gram of stomach to classify them as protected, with 1 animal showing a sterile immunity.
DISCUSSION
A major problem for development of an efficient vaccine against H. pylori in humans seems to be the choice of an appropriate mucosal adjuvant (44). Due to intestinal toxicity, the dose of heat-labile toxin had to be reduced from 10 to 5 μg in a phase I-II clinical trial, where recombinant H. pylori urease was given orally to H. pylori-infected human volunteers (33).
In the H. pylori mouse model, the use of recombinant vaccine carrier strains, such as attenuated Salmonella strains producing the UreA and UreB proteins, revealed protection in a prophylactic immunization experiment without the use of a mucosal adjuvant (6, 17). Although this approach has several advantages, one limitation might be that only a restricted number of antigens of the vaccine strain can be produced in the carrier strain. In recent safety and immunogenicity studies of phoP/phoQ-deleted Salmonella enterica serovar Typhi (11) or strain Ty21a (4) expressing H. pylori urease in adult volunteers, the vaccination was found to be safe. Although volunteers mounted an immune response against the Salmonella carrier antigens, no volunteer had detectable mucosal immune responses to the urease antigen (4, 11), but three of nine volunteers showed a weak but significant T-cell response to H. pylori urease (4).
Our study was aimed to test a further concept of antigen delivery for oral vaccination, the bacterial ghosts, which present a large number of bacterial antigens to the immune system. We hypothesized that this approach might avoid the use of adjuvant due to the rather acid- and protease-resistant nature of the bacterial ghost antigens and the presence of cell wall components with adjuvant properties. These characteristics are not found in soluble purified antigens or bacterial lysates. Thus, bacterial ghosts present a complex combination of structurally intact, rather than single, defined bacterial antigens—such as in the recombinant attenuated Salmonella approach—to the immune system.
By definition, bacterial ghosts are empty cell envelopes of gram-negative bacteria, which might be generated by expression of the bacteriophage PhiX gene E in the bacteria of choice (51). Genetic inactivation of the microorganisms leaves an intact bacterial surface. Chemical or thermal inactivation procedures may lead to the destruction of immunogenic epitopes, which might cause reduced immunogenicity (13, 34). We adapted the method here to H. pylori by expressing the E gene from a shuttle plasmid in H. pylori under the control of the λPRmut temperature-inducible promoter. We find a quantitative inactivation of the H. pylori culture, without induction of lysis-resistant bacteria. The lack of lysis-resistant mutants in H. pylori might be due to the fact that H. pylori is not a natural host of the phage PhiX, but the mechanism of this genetic inactivation in H. pylori is not understood.
Although the negative-staining electron microscopy data indicate that the cytoplasmic membrane is disrupted (Fig. 3), the disruption seems not to be efficient enough that the cytoplasmic content leaks out completely. This could be a problem with the strength of gene E expression in H. pylori, which might be reduced in comparison to that in E. coli or other bacteria. Since we do not have an antiserum against the PhiX protein we were unable to test the expression or the putative localization of the protein in H. pylori directly. To our knowledge the λPRmut promoter has not been used in H. pylori before, and nothing is known about its strength. The generation of completely empty H. pylori ghosts is still a further aim in our laboratory. This might be attained by stronger expression of the gene E or by coexpression of a DNase in H. pylori, which would degrade the viscous bacterial DNA to allow a complete emptying of the ghosts.
We used here for the first time H. pylori ghosts for vaccination experiments in the H. pylori mouse model. Although several gram-negative bacteria have been used successfully to generate bacterial ghosts, only a limited number of vaccination experiments have been performed with this type of antigen presentation. When pigs were vaccinated intramuscularly with a dose of 5 × 109 CFU of A. pleuropneumoniae ghosts or formalin-inactivated bacteria, colonization of the respiratory tract after challenge with A. pleuropneumoniae was only prevented in the ghost-vaccinated group (19).
In our experiments, we observed a clear reduction of the bacterial load in both independent vaccination experiments compared to the load in the PBS-vaccinated control (Fig. 4). A reduction of approximately 1.5 to 2 logs is considered protective vaccination, since sterile immunity is usually not obtained in the H. pylori mouse vaccination models (45). It has been shown in several studies in Helicobacter mouse models that without coadministration of a mucosal adjuvant, such as CT, LT, or mutant LT K63, to purified H. pylori proteins or whole-cell sonicates, no protective response was observed (7, 27, 29, 30). In our first experiment with H. pylori ghosts for vaccination, we obtained a reduction in the H. pylori bacterial load that is in the same range or even better than that reported for vaccination experiments with recombinant Salmonella producing H. pylori urease (1) or H. pylori sonicate plus CT (45). If we coadminister H. pylori ghosts with CT, the colonization rate can be further reduced, culminating in a sterile immunity in 3 out of 18 animals (Fig. 4).
The second vaccination experiment resulted in clearly less efficient protection in the ghost group. Since the procedure was the same as for the first experiment and the positive and negative controls (sham infected and ghosts plus CT) were comparable to those in the first experiment, it might be that the quality of the ghosts was not as high as that for the first experiment. This would indicate that the expression system has to be optimized for H. pylori, and the lysis procedure has to be controlled more rigorously to ensure that a consistent form of ghosts is produced.
In our experiments, we employed only a fixed time window for challenge and reisolation of the bacteria, and a high dose of antigen was used. As demonstrated recently by Sutton et al. (45), the dose of antigen and the time points of vaccination are important parameters that have a consequence on the vaccination result. Thus, our initial data indicate that H. pylori ghosts have to be considered as an interesting alternative to the vaccination techniques used so far to control the H. pylori infection. In future experiments, it will be necessary to optimize the vaccination parameters carefully for H. pylori ghosts. By optimizing gene expression of the E gene in H. pylori and by improving the treatment conditions after lysis, it might be feasible to generate completely empty ghosts of H. pylori. Furthermore, it will be necessary to examine the possible induction of postimmunization gastritis in vaccinated animals, as well as look for key parameters of humoral and cellular immune response elicited by the ghost vaccine.
Acknowledgments
We thank B. P. Burns for critical reading of the manuscript. We are grateful for the receipt of the mouse-adapted H. pylori strain by H. Kleanthous, OraVax, Inc.
This work was supported by EVAX Technologies, Munich, Germany, and the Deutsche Forschungsgemeinschaft (grant HA 2697/4-1 to R.H.).
Editor: D. L. Burns
REFERENCES
- 1.Aebischer, T., S. Laforsch, R. Hurwitz, F. Brombacher, and T. F. Meyer. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor α chain status and gender of infected mice. Infect. Immun. 69:556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bereswill, S., U. Waidner, S. Odenbreit, F. Lichte, F. Fassbinder, G. Bode, and M. Kist. 1998. Structural, functional and mutational analysis of the pfr gene encoding a ferritin from Helicobacter pylori. Microbiology 144:2505-2516. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt, T. G., W. D. Roof, and R. Young. 2000. Genetic evidence that the bacteriophage phi X174 lysis protein inhibits cell wall synthesis. Proc. Natl. Acad. Sci. USA 97:4297-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bumann, D., W. G. Metzger, E. Mansouri, O. Palme, M. Wendland, R. Hurwitz, G. Haas, T. Aebischer, B. von Specht, and T. F. Meyer. 2001. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 20:845-852. [DOI] [PubMed] [Google Scholar]
- 5.Corthésy-Theulaz, I., N. Porta, M. Glauser, E. Saraga, A. C. Vaney, R. Haas, J. P. Kraehenbuhl, A. L. Blum, and P. Michetti. 1995. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology 109:115-121. [DOI] [PubMed] [Google Scholar]
- 6.Corthésy-Theulaz, I. E., S. Hopkins, D. Bachmann, P. F. Saldinger, N. Porta, R. Haas, Y. Zheng-Xin, T. Meyer, H. Bouzourène, A. L. Blum, and J. P. Kraehenbuhl. 1998. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect. Immun. 66:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czinn, S. J., A. Cai, and J. G. Nedrud. 1993. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11:637-642. [DOI] [PubMed] [Google Scholar]
- 8.Czinn, S. J., and J. G. Nedrud. 1991. Oral immunization against Helicobacter pylori. Infect. Immun. 59:2359-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehio, C., and M. Meyer. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 179:538-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 11.DiPetrillo, M. D., T. Tibbetts, H. Kleanthous, K. P. Killeen, and E. L. Hohmann. 1999. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449-459. [DOI] [PubMed] [Google Scholar]
- 12.Doidge, C., I. Gust, A. Lee, F. Buck, S. Hazell, and U. Manne. 1994. Therapeutic immunisation against Helicobacter infection. Lancet 343:914-915. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, M., D. J. Wood, and P. D. Minor. 1993. Antigenic structure of poliovirus in inactivated vaccines. J. Gen. Virol. 74:685-690. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero, R. L., J.-M. Thiberge, M. Huerre, and A. Labigne. 1994. Recombinant antigens prepared from the urease subunits of Helicobacter spp.: evidence of protection in a mouse model of gastric infection. Infect. Immun. 62:4981-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, W., D. Schwan, E. Gerland, G. E. Erlenfeld, S. Odenbreit, and R. Haas. 1999. A plasmid-based vector system for the cloning and expression of Helicobacter pylori genes encoding outer membrane proteins. Mol. Gen. Genet. 262:501-507. [DOI] [PubMed] [Google Scholar]
- 16.Forman, D., D. G. Newell, F. Fullerton, J. W. G. Yarnell, A. R. Stacey, N. Wald, and F. Sitas. 1991. Association between infection with Helicobacter pylori and risk of gastric cancer—evidence from a prospective investigation. Br. Med. J. 302:1302-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Duarte, O. G., B. Lucas, Z. X. Yan, K. Panthel, R. Haas, and T. F. Meyer. 1998. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 16:460-471. [DOI] [PubMed] [Google Scholar]
- 18.Haas, R., T. F. Meyer, and J. P. M. van Putten. 1993. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol. Microbiol. 8:753-760. [DOI] [PubMed] [Google Scholar]
- 19.Hensel, A., V. Huter, A. Katinger, P. Raza, C. Strnistschie, U. Roesler, E. Brand, and W. Lubitz. 2000. Intramuscular immunization with genetically inactivated (ghosts) Actinobacillus pleuropneumoniae serotype 9 protects pigs against homologous aerosol challenge and prevents carrier state. Vaccine 18:2945-2955. [DOI] [PubMed] [Google Scholar]
- 20.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 21.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Borén. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 22.Jechlinger, W., M. P. Szostak, A. Witte, and W. Lubitz. 1999. Altered temperature induction sensitivity of the lambda pR/cI857 system for controlled gene E expression in Escherichia coli. FEMS Microbiol. Lett. 173:347-352. [DOI] [PubMed] [Google Scholar]
- 23.Kato, M., Y. Yamaoka, J. J. Kim, R. Reddy, M. Asaka, K. Kashima, M. S. Osato, F. A. K. El-Zaatari, D. Y. Graham, and D. H. Kwon. 2000. Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob. Agents Chemother. 44:2214-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotloff, K. L., M. B. Sztein, S. S. Wasserman, G. A. Losonsky, S. C. DiLorenzo, and R. I. Walker. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labigne, A., V. Cussac, and P. Courcoux. 1991. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J. Bacteriol. 173:1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Lee, A., and M. Chen. 1994. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 62:3594-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, C. K., R. Weltzin, W. D. Thomas, H. Kleanthous, T. H. Ermak, G. Soman, J. E. Hill, S. K. Ackerman, and T. P. Monath. 1995. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J. Infect. Dis. 172:161-172. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 30.Marchetti, M., M. Rossi, V. Giannelli, M. M. Giuliani, M. Pizza, S. Censini, A. Covacci, P. Massari, C. Pagliaccia, R. Manetti, J. L. Telford, G. Douce, G. Dougan, R. Rappuoli, and P. Ghiara. 1998. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine 16:33-37. [DOI] [PubMed] [Google Scholar]
- 31.Michetti, P., I. Corthesy-Theulaz, C. Davin, R. Haas, A. C. Vaney, M. Heitz, J. Bille, J. P. Kraehenbuhl, E. Saraga, and A. L. Blum. 1994. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology 107:1002-1011. [DOI] [PubMed] [Google Scholar]
- 32.Michetti, P., and R. Haas. 1994. Steps towards a vaccine, p. 53-57. In P. Malfertheiner, F. Megraud, P. Michetti, and A. Price (ed.), The year in Helicobacter pylori 1994. Current Science, Ltd., London, United Kingdom.
- 33.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 34.Nencioni, L., M. Pizza, M. Bugnoli, T. De Magistris, A. Di Tommaso, F. Giovannoni, R. Manetti, I. Marsili, G. Matteucci, D. Nucci, R. Olivieri, P. Pileri, R. Presentini, L. Villa, J. G. Kreeftenberg, S. Silvestri, A. Tagliabue, and R. Rappuoli. 1990. Characterization of genetically inactivated pertussis toxin mutants: candidates for a new vaccine against whooping cough. Infect. Immun. 58:1308-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura, A., G. N. Stemmermann, P. H. Chyou, I. Kato, G. I. Perez-Perez, and M. J. Blaser. 1991. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 325:1132-1136. [DOI] [PubMed] [Google Scholar]
- 36.Odenbreit, S., J. Püls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 37.Odenbreit, S., M. Till, D. Hofreuter, G. Faller, and R. Haas. 1999. Genetic and functional characterisation of the alpAB gene locus essential for adhesion of Helicobacter pylori to human gastric tissue. Mol. Microbiol. 31:1537-1548. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Schmitt, W., S. Odenbreit, D. Heuermann, and R. Haas. 1995. Cloning of the Helicobacter pylori recA gene and functional characterization of its product. Mol. Gen. Genet. 248:563-572. [DOI] [PubMed] [Google Scholar]
- 40.Schutze, K., E. Hentschel, B. Dragosics, and A. M. Hirschl. 1995. Helicobacter pylori reinfection with identical organisms: transmission by the patients' spouses. Gut 36:831-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sipponen, P. 1992. Natural history of gastritis and its relationship to peptic ulcer disease. Digestion 51:70-75. [DOI] [PubMed] [Google Scholar]
- 42.Spurr, A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-43. [DOI] [PubMed] [Google Scholar]
- 43.Stolte, M., and S. Eidt. 1993. Healing gastric MALT lymphomas by eradicating H. pylori. Lancet 342:568. [DOI] [PubMed] [Google Scholar]
- 44.Sutton, P., and A. Lee. 2000. Review article: Helicobacter pylori vaccines—the current status. Aliment. Pharmacol. Ther. 14:1107-1118. [DOI] [PubMed] [Google Scholar]
- 45.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 46.Szostak, M. P., A. Hensel, F. O. Eko, R. Klein, T. Auer, H. Mader, A. Haslberger, S. Bunka, G. Wanner, and W. Lubitz. 1996. Bacterial ghosts: non-living candidate vaccines. J. Biotechnol. 44:161-170. [DOI] [PubMed] [Google Scholar]
- 47.Taylor, D. N., and M. J. Blaser. 1991. The epidemiology of Helicobacter pylori infection. Epidemiol. Rev. 13:42-59. [DOI] [PubMed] [Google Scholar]
- 48.Trebesius, K., K. Panthel, S. Strobel, K. Vogt, G. Faller, T. Kirchner, M. Kist, J. Heesemann, and R. Haas. 2000. Rapid and specific detection of Helicobacter pylori macrolide resistance in gastric tissue by fluorescent in situ hybridisation. Gut 46:608-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentine, R. C., B. M. Shapiro, and E. R. Stadtman. 1968. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry 7:2143-2152. [DOI] [PubMed] [Google Scholar]
- 50.Weltzin, R., H. Kleanthous, F. Guirakhoo, T. P. Monath, and C. K. Lee. 1997. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine 15:370-376. [DOI] [PubMed] [Google Scholar]
- 51.Witte, A., G. Wanner, U. Blasi, G. Halfmann, M. Szostak, and W. Lubitz. 1990. Endogenous transmembrane tunnel formation mediated by φX174 lysis protein E. J. Bacteriol. 172:4109-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]