Abstract
The immunogenicity of a plasmid DNA vaccine incorporating Sindbis virus RNA replicase functions (pSINCP) and expressing antigen 85A (Ag85A) from Mycobacterium tuberculosis was compared with a conventional plasmid DNA vector encoding Ag85A. pSINCP-85A was highly immunogenic in mice and gave enhanced long-term protection against M. tuberculosis compared with the conventional vector.
Tuberculosis (TB) remains a significant worldwide public health problem. Despite the staggering global impact of this disease, the effectiveness of the present vaccine, Mycobacterium bovis BCG, remains uncertain; in various studies, its efficacy has varied from 0 to 80%. Clearly, there remains an urgent need for a new and more reliable TB vaccine.
Recently, there has been increasing interest in using plasmid DNA vaccination to prevent infectious diseases that require cell-mediated protective responses, including TB. While DNA vaccination has been shown to induce broad humoral and cellular immune responses in mice, it is far less immunogenic in primates and humans. Reasons for this inconsistency may include qualitative and quantitative differences in the host response and the amount of DNA injected in mice and humans.
We sought to improve upon the efficacy of conventional DNA vaccines against TB by using a DNA vaccine based on the Sindbis virus RNA replicase and encoding antigen 85A (Ag85A) from Mycobacterium tuberculosis. Incorporation of alphavirus replicons into plasmid DNA vectors to direct the amplification of RNA expressing the gene of interest has been an exciting approach toward improving DNA vaccination (8).
Vectors encoding the alphavirus-derived RNA replicase have been shown to be immunogenic in murine models at doses up to 1,000-fold lower than those used for conventional plasmid vectors (11, 16) and are effective when used as vaccines against cancer (7, 12, 15, 16) or viral infections (3, 5, 11, 14). The first product of a plasmid DNA replicon is the RNA replicase, which uses the primary positive-strand RNA transcript as a template to make negative-strand RNA and then makes more copies of full-length positive-strand RNA (encoding both replicase and antigen) as well as a shorter subgenome-length mRNA encoding only the antigen (17).
The enhanced immunogenicity of plasmid DNA replicons cannot be accounted for based only on levels of antigen production but rather through other mechanisms. In addition to producing antigen, cells transfected with the plasmid DNA replicon produce double-stranded RNA (dsRNA), which may provide immunostimulatory adjuvant effects (18). dsRNA is recognized by Toll-like receptor 3 on antigen-presenting cells and induces production of alpha interferon (IFN-α), IFN-β, IFN-α/β, interleukin 6, interleukin 12, and tumor necrosis factor alpha (2). Furthermore, replicase-based RNA and DNA vaccines have been shown to induce caspase-dependent apoptosis in transfected cells (16, 19), which can increase uptake of transfected cells by dendritic cells (19). In the context of this environment, which would likely support dendritic cell maturation (6), uptake of apoptotic transfected cells could lead to an immunogenic signal (9) and could facilitate antigen processing on presentation on major histocompatibility complex class I (1). Thus, alphavirus plasmid replicons may offer both quantitative and qualitative advantages compared with conventional plasmid vectors.
Previous studies have shown that immunization with relatively high concentrations of conventional plasmid DNA expressing the secreted form of Ag85A induces considerable anti-TB protective immunity (4). To construct a conventional plasmid DNA vector expressing Ag85A, the Ag85A gene from M. tuberculosis was cloned into the plasmid vector pcDNA3. Briefly, the Ag85A gene fused at the N terminus with a tissue plasminogen activator leader sequence was amplified by PCR using primers containing the BamHI site. Amplified DNA was digested with BamHI and ligated to BamHI-digested pcDNA3. The orientation of the Ag85A gene insert was confirmed by DNA sequencing. To construct a plasmid DNA replicon expressing Ag85A, the Ag85A gene and tissue plasminogen activator leader sequence were cloned into the plasmid vector pSINCP (Chiron, unpublished data). Plasmid pSINCP is a modified DNA-based replicon analogous to those constructs published previously (8, 11) but with alphavirus components derived from the human dendritic cell-tropic strain of Sindbis virus (10). The Ag85A gene was amplified by PCR using primers containing XbaI and PmlI sites. Amplified DNA was digested with XbaI and PmlI and ligated to XbaI- and PmlI-digested pSINCP. Recombinant plasmid DNA was prepared commercially to contain less than 100 endotoxin units per mg of DNA (Althea) and was stored at −80°C.
Female C57BL/6 mice (6 to 8 weeks old) were purchased from Charles River Laboratories. Groups of three to five mice each were injected subcutaneously with different doses of DNA diluted in sterile phosphate-buffered saline (PBS) or with BCG (Pasteur strain) in a total volume of 100 μl. Mice received an aerosol challenge with the M. tuberculosis Erdman strain either 28 days or 3 months after receiving the final DNA or BCG inoculation. For the challenge, a frozen ampoule of M. tuberculosis was thawed, briefly vortexed, and then diluted in 0.04% Tween 80-saline. During a 30-min exposure period in a Middlebrook chamber, 200 to 500 CFU of M. tuberculosis was introduced into the lung (Glas Col). Five mice were sacrificed following the challenge to confirm the dose.
Infected animals were sacrificed 28 days after aerosol challenge, and lungs and spleens were homogenized in 5 ml of PBS-0.5% Tween 80 using a Seward stomacher 80 blender (Tekmar). Tissue homogenates were diluted serially in PBS-0.5% Tween 80 and were plated on selective Middlebrook 7H11 agar. Colonies were enumerated after 2 to 3 weeks' incubation at 37°C. Statistical significance between groups was determined by analysis of variance.
To evaluate vaccine-induced cellular immune responses, mice were sacrificed 2 weeks after the second vaccination and splenocytes from vaccinated and control mice were cultured with 5 μg of purified Ag85A from M. tuberculosis (Colorado State University) per ml and 2 μg of anti-CD28 (BD PharMingen) per ml. After 3 days, supernatants were harvested. A sandwich enzyme-linked immunosorbent assay (ELISA) kit (BD PharMingen) was used to measure murine IFN-γ in culture supernatants.
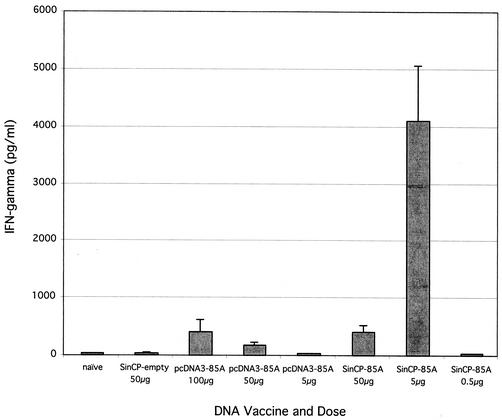
Splenocytes from mice vaccinated with pSINCP-85A at low doses secreted high levels of IFN-γ when they were restimulated in vitro with Ag85A protein for 72 h, as determined by ELISA (Fig. 1). It has previously been shown that replicon-based DNA vaccines can be highly immunogenic even at low doses, compared with conventional DNA vaccines, while both DNA formats exhibit similar immunogenicity at the highest doses. Consistent with this observation, in three separate dose titration studies, maximal IFN-γ production from mice vaccinated with pSINCP-85A was observed at the relatively low dose of between 2 and 5 μg and was dramatically higher than the maximal IFN-γ production induced by vaccination with the conventional DNA vaccine (pcDNA3-85A). For the higher doses of pSINCP-85A (20 or 50 μg), maximal IFN-γ production was similar to that seen with pcDNA3-85A at its optimal dose of 100 μg. At the lowest dose of 0.5 μg, IFN-γ production was not significantly above the background level of naïve animals.
FIG. 1.
The plasmid DNA replicon pSINCP-85A is highly immunogenic at low doses. Mice were vaccinated subcutaneously with various doses of plasmid DNA, twice, 2 weeks apart; 2 weeks after the second DNA immunization, five mice from each group were sacrificed, and spleens were removed for in vitro cytokine secretion studies. Splenocytes were stimulated with purified Ag85A protein and anti-CD28. After 72 h, supernatants were harvested and analyzed by sandwich ELISA for IFN-γ production. Data represent the mean in vitro IFN-γ production by splenocytes from individual mice plus or minus standard error and are representative of three separate studies.
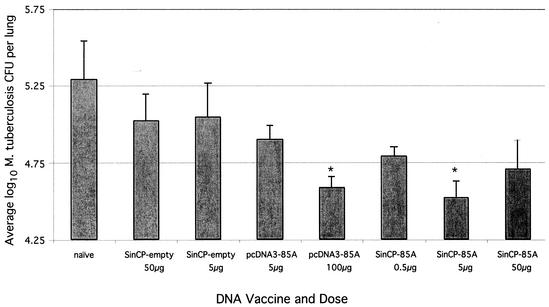
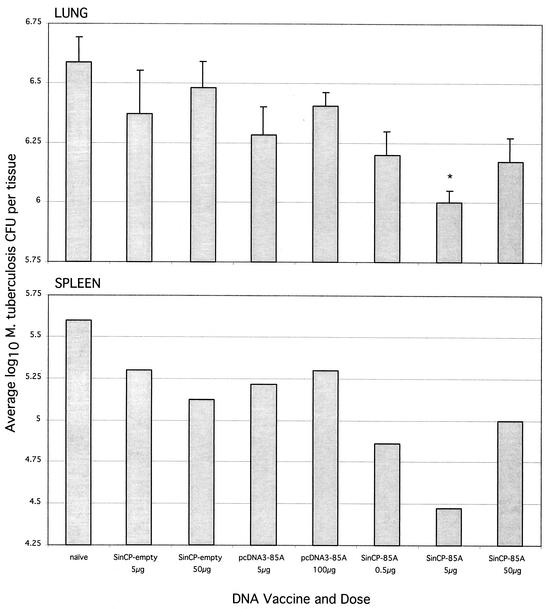
Mice were challenged with virulent M. tuberculosis 4 weeks after the second vaccination and were sacrificed 4 weeks later (Fig. 2). A 0.70 log10 reduction in bacterial counts in the lung was observed in animals vaccinated with the conventional plasmid pcDNA3-85A at a dose of 100 μg (P < 0.05) but not at the lower dose of 5 μg; however, mice receiving the plasmid DNA replicon, pSINCP-85A, at the 5-μg dose had a reduction (0.77 log10) in bacterial load of the lung similar to that in mice receiving 100 μg of conventional plasmid (P < 0.05). Reflecting the pattern observed with in vitro IFN-γ following pSINCP-85A vaccination at different doses, the 5-μg dose seemed to confer more protection than either the 50- or 0.5-μg dose. The differences between these groups did not reach statistical significance. An identical pattern was observed in the spleen, where vaccination with 5 μg of pSINCP-85A and with 100 μg of pcDNA3-85A conferred the highest level of protection (data not shown). Protection observed after 4 weeks of vaccination with both plasmid DNA vaccines tested was less than that observed with BCG vaccination (1.22 log10). To examine the long-term efficacy of the pSINCP-85A plasmid, mice were infected 12 weeks after the second DNA boost (Fig. 3). Mice were sacrificed 4 weeks postinfection, and the bacterial load was assessed in the lungs and spleen. Although the effectiveness of the conventional plasmid vector pcDNA3-85A was limited at this time point (at the 100-μg dose, there was a 0.18 log10 reduction compared with the load in nonvaccinated animals), the plasmid DNA replicon pSINCP-85A still remained efficacious. A reduction of 0.59 log10 CFU was observed in the lung (P < 0.001), and a >1 log10 CFU decrease was detected in the spleen compared with the result in nonvaccinated control animals. Mice vaccinated with BCG still had a reduction of >1 log10 in their lungs and spleen when infected 12 weeks after vaccination (data not shown).
FIG. 2.
When mice were challenged with M. tuberculosis 4 weeks after vaccination with optimal doses of either the plasmid DNA replicon pSINCP-85A or the conventional plasmid pcDNA3-85A, a similar degree of protection was observed. Mice were vaccinated subcutaneously with various doses of plasmid DNA, twice, 2 weeks apart; 4 weeks after the second DNA immunization, mice were infected by aerosol with M. tuberculosis. Four weeks after infection, five mice from each group were sacrificed, and lungs were removed for viable bacterial counts. Data points represent the geometric mean number of CFU per lung from three to five mice plus or minus standard error. *, P < 0.05 compared with naïve mice.
FIG. 3.
The plasmid DNA replicon pSINCP-85A confers long-term protection against M. tuberculosis challenge. Mice were vaccinated subcutaneously with various doses of plasmid DNA, twice, 2 weeks apart; 12 weeks after the second DNA immunization, mice were infected by aerosol with M. tuberculosis. After infection (4 weeks), mice were sacrificed, and lungs and spleens were removed for viable bacterial counts. Data points represent the geometric mean number of CFU per lung from five mice and the geometric mean number of CFU per spleen from three mice plus or minus standard error. *, P < 0.001 compared with naïve and pcDNA3-85A (100-μg) groups.
These data show that, despite substantially increased IFN-γ production relative to the levels induced by conventional DNA plasmids, immunization with pSINCP-85A at its optimal dose did not result in increased protection against the early virulent M. tuberculosis challenge 4 weeks postvaccination. A similar result has been reported for a study examining the effect of codelivery of a vector expressing granulocyte-macrophage colony-stimulating factor with vectors expressing mycobacterial antigens. In that study, although in vitro measurements of IFN-γ production by T cells showed enhancement following vaccination, the addition of the granulocyte-macrophage colony-stimulating factor-expressing plasmid vector did not impart any additional protection against an aerosol challenge with M. tuberculosis (13). This failure to augment protective immunity despite an elevated IFN-γ response may have been due to changes in the timing or location of the IFN-γ production. Alternatively, it may be that stimulation of additional factors or cell types will be necessary to improve upon protection. Of note is the finding that mice immunized with the plasmid DNA replicon pSINCP-85A have a decreased mycobacterial load compared with mice immunized with a conventional plasmid DNA vector when infected 12 weeks postvaccination. Although this appears to correlate with the enhanced IFN-γ response, in testing other DNA-based vaccines we have found that in vitro antigen-driven IFN-γ production does not always correlate with long-term protection (J. R. Kirmand and R. A. Seder, unpublished data).
These results demonstrate the efficacy and long-term durability of a novel alphavirus-derived plasmid DNA replicon, pSINCP-85A, as a vaccine against M. tuberculosis. Because one factor limiting the efficacy of DNA vaccines in primates and humans is the amount of DNA that physically can be injected, the ability of pSINCP-85A to be highly immunogenic at much lower doses of DNA might allow for enhanced efficacy in humans when compared with conventional DNA vaccines. Furthermore, the ability of pSINCP-85A to enhance innate immunity through formation of dsRNA should provide a conserved pathway in humans similar to that in rodents that may enhance cellular immune responses. Further studies are presently under way to improve the efficacy of alphavirus-derived plasmid DNA replicons as vaccines against TB. These studies include assessing the efficacy of pSINCP expressing other mycobacterial antigens.
Editor: T. R. Kozel
REFERENCES
- 1.Albert, M. L., S. F. A. Pearce, L. M. Francisco, B. Sauter, P. Roy, R. L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via alpha v beta 5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, C., P. Liljestrom, S. Ståhl, and U. F. Power. 2000. Protection against respiratory syncytial virus (RSV) elicited in mice by plasmid DNA immunisation encoding a secreted RSV G protein-derived antigen. FEMS Immunol. Med. Microbiol. 29:247-253. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, S. L., C. D. D'Souza, I. M. Orme, M. A. Liu, K. Huygen, O. Denis, A. Tang, L. Zhu, D. Montgomery, and J. B. Ulmer. 1999. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tuber. Lung Dis. 79:251-259. [DOI] [PubMed] [Google Scholar]
- 5.Berglund, C. C., C. Smerdou, M. N. Fleeton, I. Tubulekas, and P. Liljestrom. 1998. Enhancing immune responses using suicidal DNA vaccines. Nat. Biotechnol. 16:562-565. [DOI] [PubMed] [Google Scholar]
- 6.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, W. F., C. F. Hung, C. Y. Chai, K. F. Hsu, L. He, C. M. Rice, M. Ling, and T. C. Wu. 2001. Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of Mycobacterium tuberculosis heat shock protein 70 gene to an antigen gene. J. Immunol. 166:6218-6226. [DOI] [PubMed] [Google Scholar]
- 8.Dubensky, T. W., Jr., D. A. Driver, J. M. Polo, B. A. Belli, E. M. Latham, C. E. Ibanez, S. Chada, D. Brumm, T. A. Banks, S. J. Mento, D. J. Jolly, and S. M. W. Chang. 1996. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J. Virol. 70:508-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson, T. A., J. Herndon, B. Elzey, T. S. Griffith, S. Schoenberger, and D. R. Green. 2002. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J. Immunol. 168:5589-5595. [DOI] [PubMed] [Google Scholar]
- 10.Gardner, J. P., I Frolov, S. Perri, Y. Ji, M. L. MacKichan, J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariharan, M. J., D. A. Driver, K. Townsend, D. Brumm, J. M. Polo, B. A. Belli, D. J. Catton, D. Hsu, D. Mittelstaedt, J. E. McCormack, L. Karavodin, T. W. Dubensky, Jr., S. M. W. Chang, and T. A. Banks. 1998. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J. Virol. 72:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu, K.-F., C.-F. Hung, W.-F. Cheng, L. He, L. A. Slater, M. Ling, and T.-C. Wu. 2001. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther. 8:376-383. [DOI] [PubMed] [Google Scholar]
- 13.Kamath, A. T., T. Hanke, H. Briscoe, and W. J. Britton. 1999. Co-immunization with DNA vaccines expressing granulocyte-macrophage colony-stimulating factor and mycobacterial secreted proteins enhances T-cell immunity, but not protective efficacy against Mycobacterium tuberculosis. Immunology 96:511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamrud, K. I., J. W. Hooper, F. Elgh, and C. S. Schmaljohn. 1999. Comparison of the protective efficacy of naked DNA, DNA-based Sindbis replicon, and packaged Sindbis replicon vectors expressing Hantavirus structural genes in hamsters. Virology 263:209-219. [DOI] [PubMed] [Google Scholar]
- 15.Lachman, L. B., X. M. Rao, R. H. Kremer, B. Ozpolat, G. Kiriakova, and J. E. Price. 2001. DNA vaccination against neu reduces breast cancer incidence and metastasis in mice. Cancer Gene Ther. 8:259-268. [DOI] [PubMed] [Google Scholar]
- 16.Leitner, W. W., H. Ying, D. A. Driver, T. W. Dubensky, and N. P. Restifo. 2000. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 60:51-55. [PMC free article] [PubMed] [Google Scholar]
- 17.Leitner, W. W., H. Ying, and N. P. Restifo. 2000. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polo, J. M., and T. W. Dubensky, Jr. 1998. DNA vaccines with a kick. Nat. Biotechnol. 16:517-518. [DOI] [PubMed] [Google Scholar]
- 19.Ying, H., T. Z. Zaks, R. Wang, K. R. Irvine, U. S. Kammula, F. M. Marincola, W. W. Leitner, and N. P. Restifo. 1999. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 5:823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]