Abstract
In this study, we compared the apoptotic activities of clinical and environmental isolates of Vibrio vulnificus toward macrophages in vitro and in vivo. The clinical isolates induced apoptosis in macrophage-like cells in vitro and in macrophages in vivo. This suggests that macrophage apoptosis may be important for the clinical virulence of V. vulnificus.
Many bacterial pathogens that infect mammals have developed specific traits to avoid the innate and specific immune defenses of the host (3, 6, 12, 17). A characteristic common to several invasive enteric pathogens (e.g., Shigella, Salmonella, and Yersinia species) is the ability to induce macrophage apoptosis via a type III protein secretion system (8, 9, 16, 21). Macrophage apoptosis in response to Shigella and Salmonella infections triggers severe inflammation via the action of proinflammatory cytokines (22). Yersiniae induce apoptosis in macrophages by suppressing the signaling pathway that leads to the production of proinflammatory cytokines (1, 10). Macrophage cell death may lead to either the induction or the inhibition of an inflammatory response. In studying the interaction between phagocytes and Vibrio vulnificus, most efforts have focused on the capsule (7, 11, 13, 18). Encapsulated isolates of V. vulnificus are more resistant to phagocytosis by human polymorphonuclear leukocytes and murine peritoneal macrophages than are unencapsulated isolates (4, 5, 15). However, the cytotoxic effects on phagocytes have not been clearly demonstrated. In this study, we examined the apoptotic effects of nine isolates of V. vulnificus on macrophages.
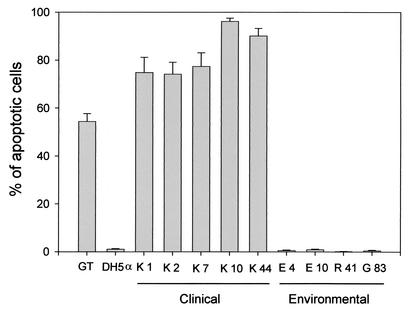
First, we examined each isolate of V. vulnificus for apoptotic activity toward a macrophage-like cell line, J774, by using terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis. Five clinical isolates of V. vulnificus, the K series of strains, were isolated from the blood of individual septicemic patients at Kurashiki Central Hospital in Japan between 1985 and 1999. Two environmental strains, E4 and E10, were isolated from seafood in Florida. Strain R41 was isolated from plankton in Okayama Prefecture in Japan. Strain G83 was isolated from seafood in the Republic of Korea. Bacteria in the logarithmic growth phase were obtained by cultivation with Luria-Bertani broth at 37°C. The desired bacterial concentration was checked by plating serial dilutions of the samples on agar and counting CFU after incubation. J774 cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL Life Technologies, Rockville, Md.) supplemented with 2 mM glutamine, 2 mM sodium pyruvate, and 20% heat-treated fetal calf serum. Cells were seeded in 24-well tissue culture plates at 105 cells/well. Each isolate of V. vulnificus was inoculated into the wells at a multiplicity of infection of approximately 1.0. After incubation at 37°C for 150 min, the Mebstain Apoptosis Kit (Immunotech, Marseilles, France) was used to label the free 3′-OH ends of DNA fragments with fluorescein as recommended by the manufacturer. The well-known apoptotic agent gliotoxin was used as the positive control (2, 14, 20). As shown in Fig. 1, all of the clinical isolates and the gliotoxin were highly apoptotic. In contrast, the ability of the environmental isolates to induce DNA fragmentations was lower than that of all of the clinical isolates. The rates of apoptosis caused by the clinical isolates ranged from 74.2 to 96.2% of the cells, whereas the rates of apoptosis caused by the environmental isolates ranged from 0.2 to 1.0%. The differences between the clinical isolates and the environmental isolates were statistically significant by the Mann-Whitney nonparametric U test (P < 0.05). Furthermore, we confirmed the formation of DNA ladders, one of the main characteristics of apoptosis (19). K44 induced the formation of DNA ladders after incubation for 80 min, whereas environmental isolates R41 and G83 did not (data not shown). However, these environmental isolates induced DNA fragmentation after further incubation, suggesting that there may be a difference in the amount of an apoptotic factor produced by these isolates. These results indicate that clinical isolates of V. vulnificus have a greater ability than environmental isolates to induce apoptosis in J774 cells in vitro.
FIG. 1.
Apoptosis was induced by clinical and environmental strains of V. vulnificus in J774 macrophage-like cells. J774 cells were coincubated with each of the clinical or environmental strains indicated for 150 min. For the positive control, cells were incubated with 20 μM gliotoxin (GT) for 4 h. Apoptotic cells were detected by the TUNEL method and by counterstaining with propidium iodide. The percentage of apoptotic cells in at least 300 cells was calculated. Data are presented as means ± standard errors and represent two independent experiments, each in triplicate wells.
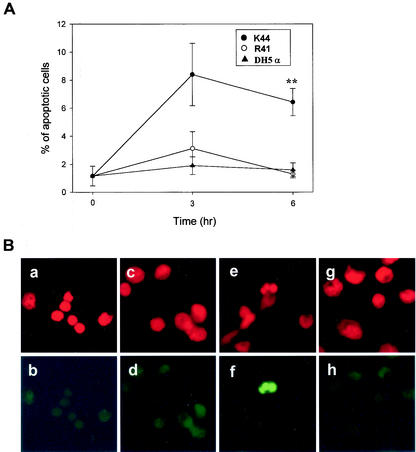
We next examined whether V. vulnificus causes apoptosis in macrophages in vivo. K44 or R41 vibrios were injected subcutaneously (s.c.) into mice. Escherichia coli DH5α was used as a negative control. Male 5-week-old ddY mice of the same colony (n = 4 for each time point) were used in this study. All of the animals used in the present study were cared for in accordance with the guidelines for animal treatment of Kitasato University, which conform to the standard principles of laboratory animal care. The bacterial cultures were washed once with phosphate-buffered saline (PBS), and 107 bacteria suspended in 0.5 ml of PBS were s.c. injected into the lower right flank of each mouse. At the times indicated (Fig. 2A), peritoneal macrophages were retrieved from the mice. Cells were resuspended in RPMI 1640 medium (Sigma Aldrich, St. Louis, Mo.) containing 100 μg of gentamicin (Sigma Aldrich) per ml and seeded in 24-well tissue culture plates with coverslips at 5 × 105 cells/well. Cells were incubated for 1 h at 37°C under 5% CO2 in air in a humidified atmosphere to allow macrophages to adhere to the glass surface, washed with RPMI 1640 medium containing 100 μg of gentamicin per ml, and further incubated for 2 h. After incubation, the cells were stained by the TUNEL method as described above. As shown in Fig. 2A, the percentage of apoptotic peritoneal macrophages from K44-infected mice was significantly increased 6 h after injection (P < 0.05). At the same time, K44 induced typical nuclear fragmentation in macrophages (Fig. 2B). On the other hand, there was no effect on the frequency of apoptosis and nuclear fragmentation in the macrophages isolated from R41-injected or DH5α-injected mice (Fig. 2A and B). It is well known that V. vulnificus bacteria evade phagocytes by means of an antiphagocytic capsule (4, 5, 15). Therefore, we confirmed the number of bacteria of each isolate in the blood and capsular expression by colony morphology and capsular staining. K44 was recovered from the blood in numbers more than 200- to 10,000-fold higher than those of R41 at 3 or 6 h after infection (data not shown), and all of our clinical isolates expressed thicker capsules than did the environmental isolates (data not shown). These data indicate that only strain K44 could escape from the host defense by means of this thicker capsular polysaccharide and could induce apoptosis in macrophages in vivo. It was not possible to determine whether R41 has the ability to induce apoptosis.
FIG. 2.
Clinical isolate K44 induces apoptosis in mouse peritoneal macrophages in vivo. Mice (n = 4 for each time point) were s.c. injected in the lower right flank with 107 bacteria suspended in 0.5 ml of PBS. Peritoneal macrophages were isolated at the indicated times after injection of bacteria. (A) Apoptotic cells were detected by the TUNEL method as described in the text. Data are presented as means ± standard errors (n = 4). **, P < 0.01 (K44 or R41 versus DH5α) according to Student's t test. (B) Nuclear morphology of mouse peritoneal macrophages stained with propidium iodide and the TUNEL method. Peritoneal macrophages were isolated at 6 h after the injection of bacteria. All of the cell nuclei in the field were stained with propidium iodide (a, c, e, and g). Apoptotic cells were stained by the TUNEL method (b, d, f, and h). Normal nuclear morphology was observed in control cells (a), in the cells of E. coli DH5α-injected mice (c), and in the cells of R41-injected mice (g). In these cases, cells were not stained by the TUNEL method (b, d, and h). In contrast, nuclear fragmentation with typical apoptotic morphology was observed in cells from mice injected with clinical isolate K44 (e and f).
We report here that V. vulnificus induces apoptosis in macrophages, as well as evading phagocytosis by macrophages, in vivo. The ability of V. vulnificus to kill macrophages by apoptosis may be important for the initiation of infection and the development of its pathogenesis.
Acknowledgments
We thank H. Sato for critical advice on the manuscript and T. Mitani, R. Shinagawa, I. Sugimoto, and M. Yoshino for technical assistance.
This work was partly supported by a grant from the School of Veterinary Medicine and Animal Sciences, Kitasato University.
Editor: J. T. Barbieri
REFERENCES
- 1.Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol. Chem. 380:795-802. [DOI] [PubMed] [Google Scholar]
- 2.Fujihara, S., C. Ward, I. Dransfield, R. T. Hay, I. J. Uings, B. Hayes, S. N. Farrow, C. Haslett, and A. G. Rossi. 2002. Inhibition of nuclear factor-kappaB activation un-masks the ability of TNF-alpha to induce human eosinophil apoptosis. Eur. J. Immunol. 32:457-466. [DOI] [PubMed] [Google Scholar]
- 3.Gobert, A. P., D. J. McGee, M. Akhtar, G. L. Mendz, J. C. Newton, Y. Cheng, H. L. Mobley, and K. T. Wilson. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl. Acad. Sci. USA 98:13844-13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson, D. E., F. M. Calia, D. M. Musher, and A. Goree. 1984. Resistance of Vibrio vulnificus to serum bactericidal and opsonizing factors: relation to virulence in suckling mice and humans. J. Infect. Dis. 150:413-418. [DOI] [PubMed] [Google Scholar]
- 5.Kreger, A., L. DeChatelet, and P. Shirley. 1981. Interaction of Vibrio vulnificus with human polymorphonuclear leukocytes: association of virulence with resistance to phagocytosis. J. Infect. Dis. 144:244-248. [DOI] [PubMed] [Google Scholar]
- 6.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298-1305. [DOI] [PubMed] [Google Scholar]
- 7.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 8.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 10.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7:180-185. [DOI] [PubMed] [Google Scholar]
- 13.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 14.Sutton, P., N. R. Newcombe, P. Waring, and A. Mullbacher. 1994. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect. Immun. 62:1192-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamplin, M. L., S. Specter, G. E. Rodrick, and H. Friedman. 1985. Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect. Immun. 49:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Velden, A. W. M., S. W. Lindgren, M. J. Worley, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinrauch, Y., D. Drujan, S. D. Shapiro, J. Weiss, and A. Zychlinsky. 2002. Neutrophil elastase targets virulence factors of enterobacteria. Nature 417:91-94. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida, S.-I., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, J. H., and M. Xu. 2000. DNA fragmentation in apoptosis. Cell Res. 10:205-211. [DOI] [PubMed] [Google Scholar]
- 20.Zhou, X., A. Zhao, G. Gopping, and P. Hirszel. 2000. Gliotoxin-induced cytotoxicity proceeds via apoptosis and is mediated by caspases and reactive oxygen species in LLC-PK1 cells. Toxicol Sci. 54:194-202. [DOI] [PubMed] [Google Scholar]
- 21.Zychlinsky, A., B. Kenny, R. Menard, M. C. Prevost, I. B. Holland, and P. J. Sansonetti. 1994. ipaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619-627. [DOI] [PubMed] [Google Scholar]
- 22.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]