Abstract
To assess the importance of two separate antioxidant activities in Helicobacter pylori, we tested the abilities of strains with mutations in either tpx (encoding thiolperoxidase) or ahpC (encoding alkyl hydroperoxide reductase [AhpC]) to colonize the stomachs of mice. The tpx strain was clearly more sensitive than the parent strain to both oxygen and cumene hydroperoxide. The strain colonized only 5% of the inoculated mice. Two different classes of oxygen-sensitive ahpC mutants in the type strain (ATCC 43504) were recently described (A. A. Olczak, J. W. Olson, and R. J. Maier, J. Bacteriol. 184:3186-3193, 2002). The same two classes of mutants were recovered upon ahpC mutagenesis of the mouse-adapted strain, SS1. Neither of these mutants was able to colonize mouse stomachs, whereas 78% of the mice inoculated with the parent strain became H. pylori positive.
Helicobacter pylori is a microaerophile that lives in the human gastric mucosa. Despite its inability to bear the oxidative stress of living in air atmospheres, it is able to tolerate the reactive oxygen stress of the host immune response. To understand what factors may be important for persistence under this stress, we constructed mutants with interruptions in genes that may be important in H. pylori's ability to deal with toxic forms of oxygen. A mutagenesis approach was used to establish a link between H. pylori's ability to dissipate reactive oxygen (via superoxide dismutase) and its ability to survive in the mouse host (15). To determine whether toxic oxygen species defense per se is a virulence determinant, we have characterized the colonization abilities for three additional antioxidant mutants.
One class of antioxidant activities that is important in protection from reactive oxygen stress is that conferred by the peroxiredoxins (6). This group of enzymes possesses a thiolperoxidase activity and can protect glutamine synthetase by preventing its peroxide-dependent oxidation. Two prominent members of this widely dispersed group are mammalian thiol-specific antioxidant and bacterial alkyl hydroperoxide reductase (AhpC). AhpC reduces organic peroxides to alcohols. Also, lipid hydroperoxides have been speculated to be substrates for the enzyme and are known to cause genotoxic effects (3). Recently, Seaver and Imlay (14) have suggested an important role for AhpC in maintenance of low intracellular hydrogen peroxide levels in Escherichia coli.
The gene ahpC (JHP 1457 [1] or HP 1563 [17]) encodes AhpC in H. pylori. Marker disruption mutagenesis of ahpC resulted in isolation of two classes of mutants (12). The predominant class of mutants (type I) was found to have increased levels of NapA (another suspected antioxidant protein), while the minor class of mutants (type II) produced parent strain levels of NapA. Both types were found to be more sensitive than the parent strain to oxidative stress-related chemicals (12). Like the peroxiredoxins, a second group of bacterial proteins, known as thiolperoxidases (Tpx) or scavengase p20s, can also use thioredoxin to reduce peroxides and protect glutamine synthetase (18, 19). The H. pylori enzyme has been purified and has a thioredoxin-linked peroxidase activity (18). Tpx is encoded by the gene tpx (also called tagD) (JHP 991 [1] or HP 0390 [17]), which is adjacent to but transcribed divergently from the gene for superoxide dismutase. Expression of tpx homologues in E. coli and Bacillus subtilis is affected by oxygen (9) and superoxide (2), respectively. E. coli tpx mutants have been shown to grow more slowly than the parent strain, and this growth defect is more pronounced in the presence of oxidative stress (4). Like the Tpx isolated from H. pylori, the enzymes isolated from E. coli, Streptococcus pneumoniae, and Haemophilus influenzae also have thioredoxin-linked peroxidase activities (5, 18). Here we assayed the sensitivity to cytotoxic agents and the colonization abilities for three phenotypically different strains of H. pylori containing mutations in tpx or ahpC.
Mutant construction.
H. pylori mutants were constructed by homologous recombination of a disrupted copy of the target gene, replacing the chromosomal copy. For each gene, mutations were made in two parental backgrounds, the type strain (ATCC 43504) (HP) and a mouse-adapted strain, SS1 (10). Construction of the HP ahpC mutants (ahpC:Kan type I and ahpC:Kan type II) was described previously (12); SS1 ahpC strains were constructed in the same way. For tpx, the gene (cloned into pBluescript KS[+]) was interrupted at its unique BstAPI site with the Campylobacter coli aphA3 gene encoding kanamycin resistance. The resultant plasmid was used to transform H. pylori. Transformants were selected for kanamycin resistance, and the genotype with an interruption of tpx was confirmed by PCR (data not shown).
Mutant characterization.
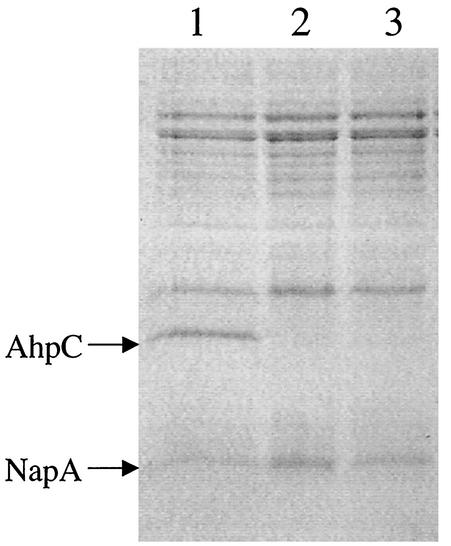
Characterization of the HP ahpC mutants has been described previously (12). Interruption of ahpC in this background resulted in two distinct classes of transformants, one with wild-type levels of NapA and one with fivefold-higher levels of NapA. We used gel electrophoresis to examine NapA levels in our SS1 ahpC mutants. As in the type strain (ATCC 43504) background, we recovered two types of ahpC mutants of SS1; AhpC− transformants that had either higher levels of NapA (type I) or wild-type levels of NapA (type II) (Fig. 1). Both of these strains were more sensitive to O2 and organic peroxides (12).
FIG. 1.
Sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis of H. pylori SS1 ahpC mutant cell extracts. Five micrograms of crude extract was loaded in each lane. Lane 1, strain SS1; lane 2, SS1 ahpC type I; and lane 3, SS1 ahpC type II. Arrows indicate AhpC (26-kDa) and NapA (17-kDa) proteins.
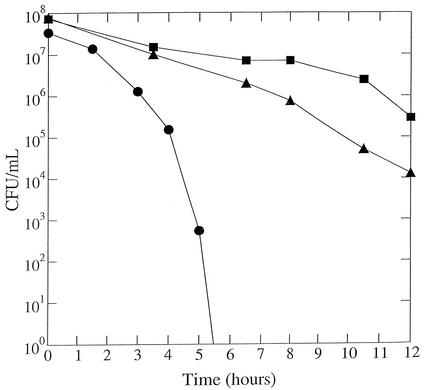
Peroxide sensitivity was measured by the disk assay. Sterile paper disks (7.5 mm in diameter) were saturated with 10 μl of one of the agents (4% [vol/vol] cumene hydroperoxide, 4% [vol/vol] t-butyl hydroperoxide, and 2 mM paraquat) resuspended in dimethyl sulfoxide. The disks were placed on 5% serum plates (100 by 15 mm, 25-ml volume) that had been previously streaked for confluent growth with wild-type or mutant cells. Plates were then placed in an incubator at 2% O2 partial pressure. Zones of growth inhibition were measured around the disks after 2 days of incubation. The distance was determined from the edge of the disk to the end of the clear zone in millimeters (12). The tpx strain exhibited a clearly increased sensitivity to cumene hydroperoxide and slightly lower sensitivity to t-butyl hydroperoxide than did its parent. The tpx strain was similar to the wild type in its sensitivity to paraquat (Table 1). In some oxidative stress characterizations the tpx strain was found to be not much different from the parent strain. For example, spontaneous rifampin resistance frequency of the tpx strain was similar to that of the parent strain (data not shown), and during a 12-h period in nongrowing conditions (15), viability of the tpx mutant was less than that of the wild type but not nearly as poor as that for a sodB mutant strain (Fig. 2).
TABLE 1.
Disk sensitivity assaya
| Strain | Size of inhibition zone for:
|
||
|---|---|---|---|
| Cumene hydroperoxideb | t-Butyl hydroperoxidec | Paraquatd | |
| Wild type | 14.7 ± 3.9 | 32.5 ± 4.1 | 2.0 ± 0.2 |
| tpx Kan | 23.7 ± 3.5 | 28.8 ± 0.2 | 2.7 ± 0.4 |
Zones of inhibition were measured (in millimeters) around filter paper disks saturated with 10 μl of the indicated compounds. Results represent the average (± standard deviation) from five independent experiments.
4% cumene hydroperoxide.
4% t-butyl hydroperoxide.
Paraquat concentration, 2 mM.
FIG. 2.
Survival of nongrowing H. pylori cells under atmospheric oxygen. Wild-type (squares), tpx mutant (triangles), or sodB mutant (circles) cells grown under 2% oxygen were suspended in PBS and incubated at 37°C under normal atmospheric conditions. Samples were removed at the times indicated in the x axis and were used for plate counts in a 2% oxygen environment.
The effect of oxygen on the growth of the tpx strain was tested on brucella agar supplemented with either 10% sheep blood or 5% horse serum. The growth sensitivity was measured by streaking wild-type and mutant strains for isolated colonies (three-way streak) and incubating them at various O2 concentrations (2, 4, 6, 8, 10, 12, and 15% O2) (12). On blood-containing medium, the mutant showed only minor growth inhibition effect by O2 when compared to the wild type. However, on serum-based medium, the tpx mutant growth was clearly more O2 inhibited than the parent strain. Blood may contain antioxidants that could mask the oxidative stress phenotype when strains are tested on these blood-containing plates. Significant growth differences between the wild type and the tpx mutant were distinguished at 10% O2 and above. At 10% O2, isolated colonies for the mutant were half the size of the wild-type colonies. At 12 and 15% partial pressure of O2, the growth of the tpx strain was significantly impaired compared to that of the wild type. Here, only slight growth at the site of the initial inoculum was present for the mutant, while the wild type showed growth at each streak. The results clearly show an oxidative stress deficiency in Tpx, and from these results combined with the known activity of the enzyme (18), we assign a role for the thiolperoxidase in combating oxidative stress.
Insertion mutagenesis by use of aphA3 was reported not to cause polar disruption (8), and the insertion of the cassette for the mutants reported here was confirmed by PCR to be within the gene of interest. Nevertheless, if the gene adjacent to ahpC (encoding a putative iron binding protein) was disrupted, that could conceivably affect the oxidative stress-related phenotype. Therefore, the AhpC mutants (T-1) were complemented successfully by introducing ahpC into the region of the H. pylori genome corresponding to HP 0405 as was described previously for complementing mutants (13). Complementation experiments on the tpx mutant were not necessary because the genes adjacent to tpx (on both sides) are transcribed in the direction opposite to that of tpx. Further, one of the genes adjacent to tpx is sodB, and, if that were affected, a much severer oxidative stress phenotype would have been observed (Fig. 2).
Mouse colonization.
Mouse colonization assays were performed essentially as described (15). Briefly, SS1 or SS1-derived mutant cells were harvested after 48 h of growth (37°C, 2% oxygen) on brucella agar (Difco) supplemented with 10% sheep blood and were suspended in phosphate-buffered saline (PBS) to an optical density at 600 nm of 1.7. Headspace in the tubes was sparged with Ar gas to minimize oxygen exposure. These suspensions were administered to C57BL/6J mice (1.5 × 108 CFU/mouse; inocula were kept constant for each experiment) via oral gavage. After 3 weeks, the mice were sacrificed and the stomachs were removed, weighed, and homogenized in Ar-sparged PBS. Homogenate was plated on brucella agar plates supplemented with bacitracin (200 μg/ml) and nalidixic acid (10 μg/ml) and was incubated for 5 to 7 days before examination for the presence of H. pylori colonies. The results of these experiments are shown in Table 2.
TABLE 2.
Mouse colonizationa of wild-type (wt) and peroxidase mutants
| Expt. designation | Colonization in:
|
|
|---|---|---|
| wt-infected mice | Mutant-infected mice | |
| 1 | 17/22 | 0/20 (tpx) |
| 2 | 8/15 | 1/14 (tpx) |
| 3 | 7/13 | 2/15 (tpx) |
| 4 | 7/12 | 0/15 (tpx) |
| 5 | 18/22 | 0/20 (ahpC T-1) |
| 6 | 14/17 | 0/17 (ahpC T-1) |
| 7 | 8/12 | 0/15 (ahpC T-2) |
Colonization is reported as the fraction of stomachs that were colonized per total number of stomachs assayed. The two ahpC strain designations T-1 and T-2 refer to type I and II phenotypes, respectively (see text and reference 12).
All three mutants were defective in colonization when compared to the parent strain. The tpx mutant had reduced ability to colonize (5% of inoculated mice were colonized), whereas both of the ahpC phenotypic mutants failed to colonize the host mice in any experiment. The latter result was seen whether NapA was upregulated or not (type I or type II strain). It has been previously shown (11, 16) that introduction of the aphA cassette into strain SS1 does not necessarily create a colonization-deficient phenotype. The inability of our oxidative stress resistance mutant strains to colonize the mouse supports the idea that oxidative stress resistance, in general, is an important factor for H. pylori virulence.
Tpx may possibly have an additional peroxide-utilizing role that augments a role in protection against reactive oxygen species. For example, a role in synthesis of surface structures that may require peroxidase-dependent assembly has been considered a possibility for Tpx of other pathogens (7). However, preliminary experiments to examine surface structures via electron microscopy have revealed no differences between our wild-type strain and our tpx mutants (data not shown).
Acknowledgments
We thank Sue Maier for expert technical assistance.
This work was funded by National Institutes of Health grant 1-RO1-DK60061-01 to R.J.M.
Editor: J. T. Barbieri
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Antelmann, H., J. Bernhardt, R. Schmid, H. Mach, U. Volker, and M. Hecker. 1997. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 18:1451-1463. [DOI] [PubMed] [Google Scholar]
- 3.Burcham, P. C. 1998. Genotoxic lipid peroxidation products: their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis 13:287-305. [DOI] [PubMed] [Google Scholar]
- 4.Cha, M.-K., H.-K. Kim, and I.-H. Kim. 1996. Mutation and mutagenesis of thiol peroxidase of Escherichia coli and a new type of thiol peroxidase family. J. Bacteriol. 178:5610-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha, M. K., H. K. Kim, and I. H. Kim. 1995. Thioredoxin-linked “thiol peroxidase” from periplasmic space of Escherichia coli. J. Biol. Chem. 270:28635-28641. [DOI] [PubMed] [Google Scholar]
- 6.Chae, H. Z., K. Robison, L. B. Poole, G. Church, G. Storz, and S. G. Rhee. 1994. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 91:7017-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes, K. J., K. D. Everiss, C. W. Harkey, and K. M. Peterson. 1994. Identification of a Vibrio cholerae ToxR-activated gene (tagD) that is physically linked to the toxin-coregulated pilus (tcp) gene cluster. Gene 148:97-100. [DOI] [PubMed] [Google Scholar]
- 8.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 9.Kim, H. K., S. J. Kim, J. W. Lee, M. K. Cha, and I. H. Kim. 1996. Identification of promoter in the 5′-flanking region of the E. coli thioredoxin-linked thiol peroxidase gene: evidence for the existence of oxygen-related transcriptional regulatory protein. Biochem. Biophys. Res. Commun. 221:641-646. [DOI] [PubMed] [Google Scholar]
- 10.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 11.Nolan, K. J., D. J. McGee, H. M. Mitchell, T. Kolesnikow, J. M. Harro, J. O'Rourke, J. E. Wilson, S. J. Danon, N. D. Moss, H. L. Mobley, and A. Lee. 2002. In vivo behavior of a Helicobacter pylori SS1 nixA mutant with reduced urease activity. Infect. Immun. 70:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olczak, A. A., J. W. Olson, and R. J. Maier. 2002. Oxidative-stress resistance mutants of Helicobacter pylori. J. Bacteriol. 184:3186-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson, J. W., N. S. Mehta, and R. J. Maier. 2001. Requirement of nickel metabolism proteins HypA and HypB for full activity of both hydrogenase and urease in Helicobacter pylori. Mol. Microbiol. 39:176-182. [DOI] [PubMed] [Google Scholar]
- 14.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyler, R. W., Jr., J. W. Olson, and R. J. Maier. 2001. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect. Immun. 69:4034-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takata, T., E. El-Omar, M. Camorlinga, S. A. Thompson, Y. Minohara, P. B. Ernst, and M. J. Blaser. 2002. Helicobacter pylori does not require Lewis X or Lewis Y expression to colonize C3H/HeJ mice. Infect. Immun. 70:3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 18.Wan, X. Y., Y. Zhou, Z. Y. Yan, H. L. Wang, Y. D. Hou, and D. Y. Jin. 1997. Scavengase p20: a novel family of bacterial antioxidant enzymes. FEBS Lett. 407:32-36. [DOI] [PubMed] [Google Scholar]
- 19.Zhou, Y., X. Y. Wan, H. L. Wang, Z. Y. Yan, Y. D. Hou, and D. Y. Jin. 1997. Bacterial scavengase p20 is structurally and functionally related to peroxiredoxins. Biochem. Biophys. Res. Commun. 233:848-852. [DOI] [PubMed] [Google Scholar]