Abstract
Splenic dendritic cells (DCs) obtained from mice at 48 h after Listeria monocytogenes infection exhibited up-regulation of CD80 and produced higher titers of gamma interferon (IFN-γ) and interleukin-12 (IL-12) than did DCs obtained from uninfected mice. Mice immunized with DCs obtained from mice that had been infected with L. monocytogenes 48 h before acquired host resistance to lethal infection with L. monocytogenes at 4 and 8 weeks. Immunization with DCs from heat-killed L. monocytogenes failed to induce resistance. Acquired antilisterial resistance is specific, since the immunized mice could not be protected from Salmonella enterica serovar Typhimurium infection. Infected DCs stimulated proliferation of naive CD4+ and CD8+ cells in vitro, suggesting that in vivo-infected DCs activate CD8+ T cells, which are critical in acquired antilisterial resistance, as well as CD4+ T cells. When wild-type mice were immunized with DCs from IFN-γ-deficient mice, they were protected against a lethal L. monocytogenes challenge. In contrast, when mice were immunized with DCs from anti-IL-12 p40 monoclonal antibody-injected mice, they failed to gain acquired antilisterial resistance. These results suggest that DC-derived IL-12, but not IFN-γ, may play a critical role in induction of acquired antilisterial resistance. Our present results suggest that splenic DCs obtained from mice infected with L. monocytogenes in vivo may be an effective immunogen with which to induce antigen-specific immunity.
The dendritic cell (DC) is a major antigen-presenting cell, and DCs efficiently activate T cells. Immature DCs are resident in the peripheral tissues and circulate in the bloodstream. When immature DCs encounter antigens, these cells take them up by phagocytosis and migrate to regional lymph nodes via afferent lymphatic vessels. In the regional lymph nodes, immature DCs mature and activate naive T cells (2). Mature DCs can produce cytokines, including interleukin-12 (IL-12), IL-18, tumor necrosis factor alpha, gamma interferon (IFN-γ), and IFN-α, and chemokines such as macrophage inflammatory proteins 1α and 1β and monocyte chemoattractant protein 1 (16, 26, 29, 31, 32).
Recent studies have indicated that in vitro antigen-pulsed DCs induce antigen-specific immunity (4, 15, 18, 20-22, 30, 34, 36). DCs pulsed with tumor-associated peptides developed a tumor-specific T-cell response and protected against a lethal tumor challenge (21). DCs infected with Chlamydia trachomatis in vitro induced an acquired antichlamydial response (30, 34). DCs pulsed in vitro with Borrelia burgdorferi induced B. burgdorferi-specific antibody production (22). Similarly, DCs pulsed with Pseudomonas aeruginosa (36), Candida albicans (4), or lymphocytic choriomeningitis virus (18) were able to induce antigen-specific responses. Antigen-pulsed mature DCs express genes that induce DC migration, activation, and recruitment of T cells (30), and different antigens phagocytosed by DCs induce different patterns of cytokine production by CD4+ T cells, depending on the cytokines produced by the DCs (4).
A Th1 response is important for induction of protective immunity against intracellular bacteria (15, 20, 22, 34). Bacterium-infected DCs secrete significant amounts of IL-12 (20), and IL-12 and IFN-γ are involved in Th1 development. IL-12 can augment IL-12 production by DCs and promotes IFN-γ production (7, 25). Listeria monocytogenes, a facultative intracellular pathogen, induces intensive cell-mediated immunity (17). L. monocytogenes infection promotes the induction of a host Th1 response, which is critical in acquired resistance against L. monocytogenes (5, 24).
We were interested in induction of acquired host resistance to L. monocytogenes infection by immunizing DCs. In vitro antigen-pulsed DCs have been generally used for immunization. However, it is possible that loss of viability sometimes occurs when DCs are pulsed with viable bacteria in vitro. Therefore, we investigated the efficiency of induction of acquired antilisterial resistance by immunization with splenic DCs isolated from L. monocytogenes-infected mice. In this paper, we report that in vivo-infected DCs are able to induce acquired resistance to a lethal L. monocytogenes infection as well as in vitro-pulsed DCs and that IL-12 may play a critical role in the induction of this acquired resistance.
MATERIALS AND METHODS
Mice.
C57BL/6 mice, IFN-γ-deficient (IFN-γ−/−) mice on a C57BL/6 × Sv129 background (35), and their wild-type (IFN-γ+/+) counterparts, 6 to 10 weeks old, were used. C57BL/6 mice were obtained from Japan SLC, Hamamatsu, Shizuoka, Japan. The animals were maintained under specific-pathogen-free conditions in the Institute for Animal Experiments, Hirosaki University School of Medicine. All of the animal experiments in the present study were carried out in accordance with the guidelines for animal experimentation of Hirosaki University.
Bacteria.
L. monocytogenes 1b 1684 (24) and Salmonella enterica serovar Typhimurium χ3306 (8, 37) cells were prepared as described previously. The concentration of washed cells was adjusted spectrophotometrically at 550 nm, and cells were stored at −80°C until use. The 50% lethal doses of an L. monocytogenes stock suspension were 5 × 105 CFU for C57BL/6 mice and 5 × 104 CFU for IFN-γ−/− mice when the mice were infected intravenously. The 50% lethal dose of an S. enterica serovar Typhimurium stock suspension was less than 50 CFU for C57BL/6 mice when the mice were infected intraperitoneally. Heat-killed L. monocytogenes (HKLM) cells were obtained by heating the bacteria in a boiling water bath for 1 h.
Preparation and enrichment of DCs from spleen tissue.
Single-cell suspensions from spleens of mice were prepared as previously described (27, 28). Briefly, spleen cells were washed twice with RPMI 1640 medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) supplemented with 2% heat-inactivated fetal calf serum (FCS; JRH Biosciences, Lenexa, Kans.). Spleen cells were then digested with 1 mg of collagenase D (Roche Molecular Biochemicals, Mannheim, Germany) per ml in RPMI 1640 medium supplemented with 5% FCS and 10 mM HEPES for 25 min at 37°C in a 5% CO2 incubator. The remaining solid tissues were forced through 100-mesh stainless steel sieves. Cells were recovered by centrifugation at 300 × g for 5 min, resuspended in a 17% Optiprep (Nycomed Pharma, Oslo, Norway) solution diluted with Ca2+- and Mg+-free Hanks balanced salt solution, overlaid with 12% Optiprep (1.068 g/ml) diluted with 0.88% (wt/vol) NaCl-1 mM EDTA-10 mM HEPES-0.5% bovine serum albumin (pH 7.4; Sigma Chemical Co., St. Louis, Mo.) and then 2 ml of Ca2+- and Mg+-free Hanks' balanced salt solution, and centrifuged at 600 × g for 15 min at 20°C. The low-density cells at the interface between the Hanks’ balanced salt solution and the 12% Optiprep solution were harvested; washed twice with RPMI 1640 medium supplemented with 2% FCS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml; and then purified by AutoMACS with CD11c microbeads (Miltenyi Biotec Inc., Auburn, Calif.). More than 95% of the recovered cells were CD11c+.
Flow cytometric analysis.
Preincubation with Fc Block (BD Biosciences, San Diego, Calif.) was carried out to prevent nonspecific binding of labeled monoclonal antibodies (MAbs). The cells were incubated for 30 min at 4°C with a fluorescein isothiocyanate-labeled anti-CD11c MAb (BD Biosciences) plus a phycoerythrin-labeled anti-CD80 MAb (BD Biosciences) or a phycoerythrin-labeled anti-I-Ab MAb (BD Biosciences). The MAb-labeled cells were analyzed with a FACScalibur flow cytometer (BD Immunocytometry Systems, San Jose, Calif.).
Immunization with DCs.
Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes or injected with 5 × 106 HKLM cells. Two days later, their spleens were aseptically removed and a spleen cell suspension was prepared. DCs were then purified from the spleen cells as described above. For the preparation of in vitro-infected DCs, purified DCs from naive mice were resuspended in RPMI 1640 medium containing 10% FCS and cultured with 10-fold numbers of viable L. monocytogenes for 30 min at 37°C in a 5% CO2 incubator. The cells were then washed twice with RPMI 1640 medium supplemented with 10% FCS, 100 U of penicillin per ml, and 100 μg of streptomycin per ml and resuspended in antibiotic-free RPMI 1640 medium. Mice were immunized intravenously with 105 or 5 × 104 in vivo-infected or in vitro-pulsed DCs. As a control, mice were immunized with uninfected DCs. As directly immunized controls, mice were immunized intravenously with 105 CFU of L. monocytogenes or with a dose of viable L. monocytogenes equivalent to that of the viable bacteria existing in 105 DCs from the infected mice. Mice were injected with phosphate-buffered saline (PBS) only as a nonimmune control.
Evaluation of acquired resistance to a lethal bacterial infection.
DC-immunized mice were challenged with 5 × 106 CFU of L. monocytogenes intravenously or 1,000 CFU of S. enterica Typhimurium intraperitoneally at 4 or 8 weeks after immunization. Their spleens and livers were aseptically removed 48 h after the challenge and homogenized in RPMI 1640 medium containing 1% 3-[(cholamidopropyl)dimethylammonio]-1-propanesulfate (CHAPS; Wako Pure Chemical Co., Osaka, Japan). The numbers of viable bacteria in the organs of infected mice were counted by plating serial 10-fold dilutions of organ homogenates on tryptic soy agar plates (BD Diagnostic Systems, Sparks, Md.) for L. monocytogenes or L-agar plates (Invitrogen Corp., Carlsbad, Calif.) for S. enterica serovar Typhimurium. Colonies were routinely counted 24 h later.
In vivo depletion of endogenous cytokines.
A hybridoma cell line secreting a MAb against mouse IL-12 p40 (C17.8; rat immunoglobulin G1) was injected into pristane-primed CD-1 nu/nu mice. Anti-IL-12 MAb found in the ascites fluid was partially purified by (NH4)2SO4 precipitation. The mice were given a single intravenous injection of 2 mg of the anti-IL-12 MAb 2 h before infection. Normal rat globulin was injected as a control. The MAb and normal rat globulin contained endotoxin at less than 0.1 ng per injected dose, as determined by use of the Limulus amoebocyte lysate assay.
Cytokine production by DCs.
DCs (105 resuspended in 100 μl of RPMI 1640 medium supplemented with 10% FCS) were stimulated with 106 HKLM cells for 24 h in a 96-well tissue culture plate (Greiner Labortechnik, Frickenhausen, Germany), and supernatants were collected and stored until cytokine assays were performed. Titers of IL-12 p70 and IFN-γ in the culture supernatants were determined by double sandwich enzyme-linked immunosorbent assays as described previously (23, 24).
T-cell proliferation assay.
Mice were infected with 5 × 105 CFU of L. monocytogenes intravenously, and spleens were removed 2 days later. Splenic DCs were purified as described above. Splenic CD4+ and CD8+ cells were purified by AutoMACS with CD4 and CD8 microbeads (Miltenyi Biotec Inc.). The purity of isolated CD4+ and CD8+ cells was more than 95%, as shown by flow cytometric analysis. CD4+ T cells, CD8+ cells, and irradiated (20 Gy) DCs were suspended in RPMI 1640 medium supplemented with 10% FCS, 10 μM sodium pyruvate (Wako), and 50 μM 2-mercaptoethanol (Wako). CD4+ T cells or CD8+ cells (25 × 104) and DCs (5 × 104) in a total volume of 100 μl were cultured with or without 0.1 μg of an anti-CD3 MAb (145-2C11; rat immunoglobulin G1) in a 96-well tissue culture plate for 48 h, and 20 kBq of [3H]thymidine (ICN Biomedicals Inc., Irvine, Calif.) per well was added. Counts per minute incorporated by harvested cells were determined with a gamma scintillation counter 24 h later.
Statistical evaluation of data.
Data were expressed as means ± standard deviations, and Student's t test was used to determine the significance of the differences in the bacterial counts and cytokine titers of the specimens and in [3H]thymidine incorporation by the control and experimental groups. Each experiment was repeated at least twice and accepted as valid only when trials showed similar results.
RESULTS
Induction of host resistance to lethal L. monocytogenes infection by immunization with in vitro L. monocytogenes-pulsed DCs.
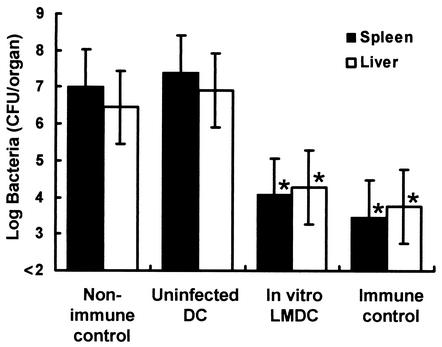
We investigated whether DCs pulsed in vitro with L. monocytogenes can induce antilisterial resistance. Spleens were removed from naive mice, and CD11c+ DCs were purified from the splenocytes. The DCs were incubated with 10 CFU of viable L. monocytogenes per DC for 30 min. We found 2 × 104 to 4 × 104 CFU of viable L. monocytogenes within 105 in vitro-pulsed DCs. After killing of viable bacteria, mice were immunized intravenously with 105 DCs pulsed with L. monocytogenes in vitro or uninfected DCs. As directly immunized controls, mice were immunized intravenously with 105 CFU of L. monocytogenes. Nonimmunized mice were injected with PBS only. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h after the challenge. Bacterial numbers in the organs of mice that had been immunized with infected DCs in vitro were significantly lower than those in the organs of mice immunized with uninfected DCs (Fig. 1). The magnitude of antilisterial resistance of the mice immunized with in vitro-pulsed DCs was almost equal to that of the directly immunized mice (Fig. 1).
FIG. 1.
Acquired resistance to a lethal L. monocytogenes infection in mice immunized with in vitro L. monocytogenes-pulsed DCs. Spleens were removed from uninfected mice, and CD11c+ DCs were purified. The DCs were incubated with 10 CFU of viable L. monocytogenes per DC as described in the text. After killing of viable L. monocytogenes by antibiotics, naive mice were immunized with 105 in vitro-infected DCs (In vitro LMDC) or uninfected DCs. Nonimmune control mice were injected with PBS only. These mice were challenged intravenously with 5 × 106 CFU of L. monocytogenes 4 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. The immune control mice were directly immunized with 105 CFU of L. monocytogenes. Each result represents the mean ± the standard deviation of a group of six mice from two independent experiments. An asterisk indicates a significant difference from the value for the nonimmune control group (P < 0.01).
Flow cytometric analysis of DCs from L. monocytogenes-infected mice.
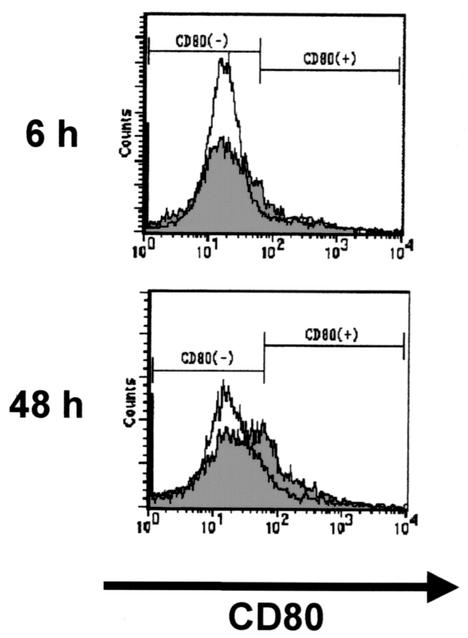
Next, we investigated whether DCs obtained from L. monocytogenes-infected mice could induce antilisterial resistance. Preliminarily, activation of DCs by infection was evaluated by measuring the expression of CD80 and I-Ab on DCs. Mice were infected with 5 × 105 CFU of L. monocytogenes, and CD11c+ cells were purified from their spleens 6 or 48 h later and analyzed for CD80 and I-Ab by flow cytometry. DCs obtained from the mice at 6 h postinfection showed almost the same level of CD80 expression as those from uninfected mice. In contrast, DCs obtained from mice at 48 h after infection exhibited significant up-regulation of CD80 expression, compared with those from infected mice (Fig. 2). I-Ab expression in DCs was comparable between the two groups (data not shown). Therefore, we chose DCs obtained at 48 h after infection for immunization.
FIG. 2.
Flow cytometric analysis of DCs from uninfected and L. monocytogenes-infected mice. Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes, and their spleens were removed 6 or 48 h later. CD11c+ DCs were prepared as described in the text. Expression of CD80 on the cell surface was analyzed by flow cytometry. The open histogram indicates the CD80 fluorescence intensity of DCs from uninfected mice, and the filled histogram indicates that of DCs from L. monocytogenes-infected mice.
Induction of host resistance to lethal L. monocytogenes infection by immunization with DCs infected in vivo with L. monocytogenes.
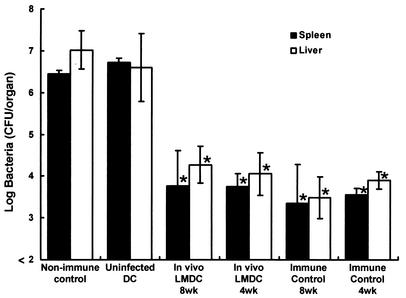
We investigated the induction of antilisterial resistance by immunization with in vivo-infected DCs. Mice were infected with 5 × 105 CFU of viable L. monocytogenes, and DCs were purified from their spleens 48 h later. Naive mice were immunized with 105 DCs. Some mice were immunized with DCs obtained from uninfected mice. Immunized control mice were injected with 105 CFU of viable L. monocytogenes, while nonimmunized control mice were injected with PBS only. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes 4 or 8 weeks later. The number of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 3). Elimination of L. monocytogenes from the organs was markedly augmented by immunization with DCs infected with L. monocytogenes in vivo. There was no difference in the efficacy of bacterial elimination from the organs between the 4- and 8-week immunizations. Moreover, the magnitudes of antilisterial resistance were similar in mice immunized with in vivo-infected DCs and mice immunized with L. monocytogenes. These results suggest that immunization with in vivo-infected DCs is effective at inducing host resistance to a lethal L. monocytogenes infection.
FIG. 3.
Acquired resistance to a lethal L. monocytogenes infection in mice immunized with in vivo L. monocytogenes-infected DCs. Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes, and their spleens were removed 48 h after infection. Naive mice were immunized with 105 DCs from L. monocytogenes-infected mice (In vivo LMDC) or from uninfected mice (Uninfected DC). Nonimmune control mice were injected with PBS only, and immune control mice were infected intravenously with 105 CFU of L. monocytogenes. They were challenged with 5 × 106 CFU of L. monocytogenes 4 or 8 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. Each result represents the mean ± the standard deviation for a group of six to nine samples from three independent experiments. An asterisk indicates a significant difference from the value for the uninfected, DC-immunized group (P < 0.01).
Antigen specificity of host resistance to a lethal L. monocytogenes infection in mice immunized with DC infected in vivo.
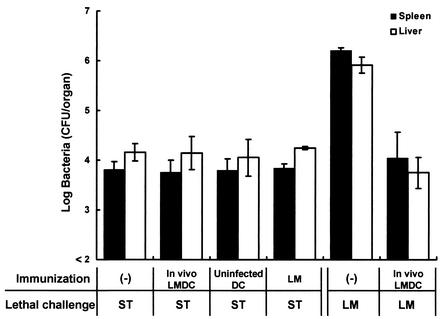
To shorten the immunization period, we intended to use 4-week immunization with in vivo-infected DCs in the following experiments. To exclude the possibility that nonspecific resistance induced by immunization may still remain at 4weeks after immunization, we estimated whether enhancement of host resistance to lethal S. enterica serovar Typhimurium infection would be observed in mice that had been immunized with in vivo-infected DCs 4 weeks before. Mice were infected with 5 × 105 CFU of viable L. monocytogenes, and DCs were purified from their spleens 48 h later. Naive mice were immunized with 105 DCs from each group. DCs obtained from uninfected mice were immunized as a control. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes or 1,000 CFU of S. enterica serovar Typhimurium 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 4). Elimination of S. enterica serovar Typhimurium from the organs of mice immunized with DCs infected with L. monocytogenes in vivo, compared with those of nonimmunized mice and mice immunized with uninfected DCs, was not augmented. On the other hand, the number of viable L. monocytogenes bacteria in the organs of mice immunized with DCs infected with L. monocytogenes in vivo was significantly decreased compared with that in the organs of nonimmunized mice. These results suggest that antilisterial resistance induced by in vivo-infected DCs is antigen specific.
FIG. 4.
Antigen specificity of host resistance to lethal L. monocytogenes infection in mice immunized with in vivo-infected DCs. Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes, and their spleens were removed 48 h later. Naive mice were immunized with 105 DCs from L. monocytogenes-infected mice (In vivo LMDC) or from uninfected mice (Uninfected DC). Nonimmune control mice were injected with PBS only, and immune control mice were infected intravenously with 105 CFU of L. monocytogenes. They were challenged with 1,000 CFU of S. enterica serovar Typhimurium (ST) or 5 × 106 CFU of L. monocytogenes (LM) 4 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. Each result represents the mean ± the standard deviation for a group of six mice from two independent experiments.
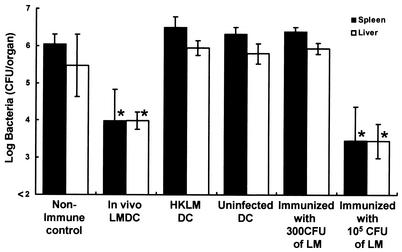
Failure of immunization with DCs injected in vivo with HKLM to induce host resistance to a lethal L. monocytogenes infection.
We investigated whether antilisterial resistance can be induced by immunization with DCs from mice injected with HKLM. Mice were infected with 5 × 105 CFU of viable L. monocytogenes or injected with 5 × 106 CFU of HKLM, and DCs were purified from their spleens 48 h later. Naive mice were immunized with 105 DCs from each group. DCs obtained from uninfected mice were used for control immunization. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 5). Elimination of bacteria from the organs was significantly augmented by immunization with in vivo-infected DCs, compared with that in mice immunized with uninfected DCs, whereas DCs from HKLM-immunized mice failed to induce resistance.
FIG. 5.
Resistance to lethal L. monocytogenes infection in mice immunized with DCs from mice injected with HKLM. Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes or 5 × 106 HKLM cells, and their spleens were removed 48 h later. Naive mice were immunized with 105 DCs from L. monocytogenes-infected mice (In vivo LMDC), from HKLM-injected mice (HKLM DC), or from uninfected mice (Uninfected DC). Nonimmune control mice were injected with PBS only. They were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. Two directly immunized controls were employed. The first immune group was immunized with 300 CFU of L. monocytogenes, which is equivalent to the bacterial number found in 105 of the infected DCs used for immunization. The second immune group was immunized with 105 CFU of L. monocytogenes. Each result represents the mean ± the standard deviation for a group of six mice from two independent experiments. An asterisk indicates a significant difference from the value for the nonimmune control group (P < 0.01).
Failure of immunization with DC-harboring viable bacteria to induce host resistance to a lethal L. monocytogenes infection.
In our present study, 200 to 300 CFU of viable L. monocytogenes were usually detected at 48 h postinfection in 105 purified DCs obtained from mice that had been infected with 5 × 105 CFU of bacteria. It is possible that antilisterial resistance is induced by viable L. monocytogenes existing in in vivo-infected DCs. To address this possibility, we compared the host resistance to lethal L. monocytogenes infection of mice immunized with 300 CFU of bacteria with that of mice immunized with 105 CFU of bacteria. They were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 5). Bacterial numbers in the organs of mice that had been immunized with 300 CFU of bacteria were comparable to those in the organs of nonimmune control mice, whereas elimination of bacteria from the organs of mice immunized with 105 CFU of bacteria was markedly enhanced as well as in those of mice immunized with in vivo-infected DCs. These results suggest that induction of host resistance by immunization with in vivo-infected DCs is not due to viable L. monocytogenes harbored in the DCs.
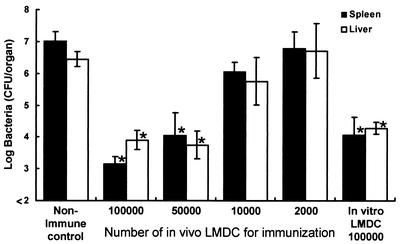
Dependence of acquisition of host resistance to a lethal L. monocytogenes infection on the number of DCs used for immunization.
Next, we investigated the dependence of acquisition of antilisterial resistance on the number of in vivo-infected DCs used for immunization. Mice were infected with 5 × 105 CFU of viable L. monocytogenes, and DCs were purified from their spleens 48 h later. Naive mice were immunized with 105, 5 × 104, 104, or 2 × 103 in vivo-infected DCs, respectively. To compare the immunizing ability of DCs infected in vivo with that of DCs pulsed in vitro, mice were immunized with 105 in vitro-pulsed DCs. Nonimmune control mice were injected with PBS only. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 6). Elimination of bacteria from the organs was augmented when mice were immunized with 105 or 5 × 104 in vivo-infected DCs, whereas antilisterial resistance was not acquired when mice were immunized with fewer than 104 DCs, suggesting that acquisition of antilisterial resistance induced by immunization with in vivo-infected DCs is dependent on the dose of DCs used as an immunogen. The efficacies of bacterial elimination were similar when mice were immunized with 5 × 104 in vivo-infected DCs and 105 in vitro-pulsed DCs.
FIG. 6.
Dependence of acquired resistance to a lethal L. monocytogenes infection on the number of L. monocytogenes-infected DCs (LMDC) used for immunization. Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes, and their spleens were removed 48 h later. Naive mice were immunized with 105, 5 × 104, 104, or 2 × 103 purified DCs from L. monocytogenes-infected mice or with 105 in vitro-pulsed DCs. Nonimmune control mice were injected with PBS only. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes intravenously 4 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. Each result represents the mean ± the standard deviation for a group of six mice from two independent experiments. An asterisk indicates a significant difference from the value for the nonimmune control group (P < 0.01).
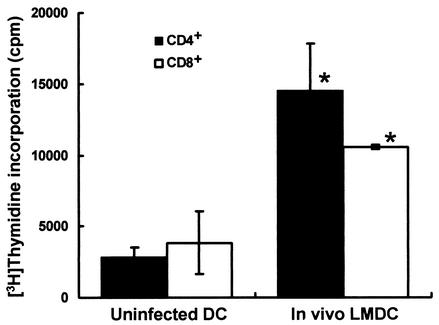
Activation of naive T cells by L. monocytogenes-infected DCs.
We investigated whether proliferation of naive T cells would be stimulated by in vivo-infected DCs. Mice were infected with 5 × 105 CFU of L. monocytogenes, and DCs were purified from their spleens 48 h later. We cultured 25 × 104 naive splenic CD4+ or CD8+ T cells with 5 × 104 irradiated DCs from L. monocytogenes-infected mice or uninfected mice in the presence of an anti-CD3 MAb and then estimated T-cell proliferation (Fig. 7). Proliferation of both naive CD4+ and CD8+ T cells was augmented when they were cultured with irradiated DCs infected with L. monocytogenes in vivo, compared with that of those cultured with uninfected DCs, suggesting that in vivo-infected DCs can stimulate proliferation of both naive CD4+ T cells and CD8+ T cells.
FIG. 7.
Proliferation of naive T cells stimulated by DCs infected with L. monocytogenes in vivo (LMDC). Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes. DCs were prepared at 48 h after infection as described in the text. Irradiated DCs (5 × 104) from L. monocytogenes-infected mice (In vivo LMDC) or from uninfected mice (Uninfected DC) were cultured with 25 × 104 CD4+ or CD8+ T cells and 0.1 μg of anti-CD3 MAb per 100 μl for 48 h and then pulsed with 20 kBq of [3H]thymidine per well for 24 h. Proliferation was determined by calculating the mean number of counts per minute ± the standard deviation in triplicate wells. An asterisk indicates a significant difference from the value for the uninfected, DC-immunized group (P < 0.01).
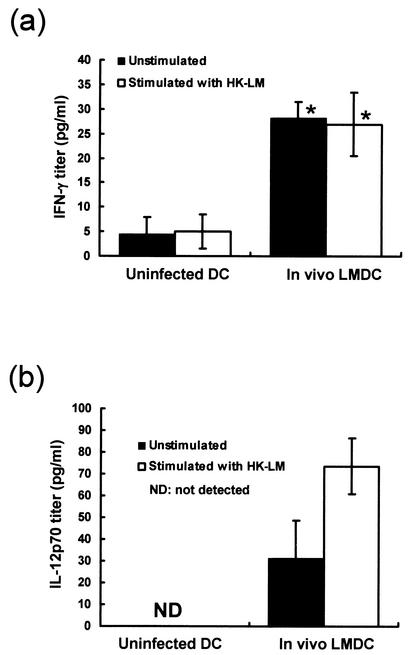
Cytokine production in DCs from L. monocytogenes-infected mice.
Mature DCs can reportedly produce IFN-γ and IL-12 p70 (13). Therefore, we investigated the production of these cytokines in DCs obtained from uninfected and L. monocytogenes-infected mice. Mice were infected with 5 × 105 CFU of L. monocytogenes, and DCs were purified from their spleens 48 h later. DCs obtained from uninfected mice were used as a control. DCs were cultured in the presence or absence of HKLM for 24 h, and titers of IFN-γ and IL-12 p70 in the supernatants were determined. DCs obtained from L. monocytogenes-infected mice produced significantly higher titers of IFN-γ than did uninfected DCs either in the presence or in the absence of HKLM (Fig. 8a). DCs obtained from L. monocytogenes-infected mice exhibited a significant level of IL-12 p70 without stimulation with HKLM, and IL-12 production was enhanced by stimulation with HKLM. In contrast, DCs from uninfected mice produced no IL-12 p70 even when stimulated with HKLM (Fig. 8b). These results suggest that DCs obtained from L. monocytogenes-infected mice may be activated and that they are able to produce cytokines that drive Th1 polarization.
FIG. 8.
Cytokine production by DCs from L. monocytogenes-infected mice. Mice were infected intravenously with 5 × 105 CFU of L. monocytogenes. Their spleens were obtained 48 h later, and CD11c+ DCs were purified as indicated in the text. DCs (105/100 μl) obtained from L. monocytogenes-infected mice (In vivo LMDC) or from uninfected mice (Uninfected DC) were cultured for 24 h in the presence or absence of 106 HKLM cells. Titers of IFN-γ (a) and IL-12 p70 (b) in the culture supernatants were determined. The results were reproduced in three repeated experiments. Each result represents the mean ± the standard deviation for a group of six to nine samples from three independent experiments. An asterisk indicates a significant difference from the value for the uninfected, DC-immunized group (P < 0.01).
Involvement of DC-derived IFN-γ in the induction of host resistance to lethal L. monocytogenes infection.
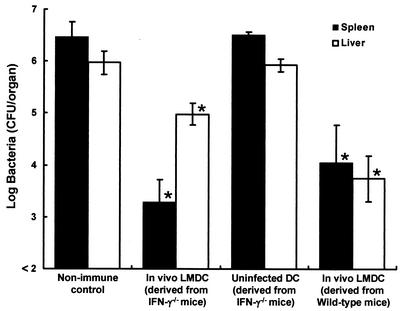
We investigated whether IFN-γ produced by L. monocytogenes-infected DCs would be critical in induction of host resistance to lethal L. monocytogenes infection. IFN-γ-deficient mice were infected with 5 × 104 CFU of L. monocytogenes, and DCs were purified from their spleens 48 h later. Naive wild-type mice were immunized with 5 × 104 DCs. Control mice were immunized with DCs obtained from uninfected IFN-γ-deficient mice. Nonimmune control mice were injected with PBS only. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 9). The number of bacteria was significantly reduced in the organs of mice that had been immunized with infected DCs obtained from IFN-γ-deficient mice, compared with that in the organs of nonimmune control mice and mice immunized with uninfected DCs. These results suggest that IFN-γ produced by activated DCs may play a marginal role in the induction of acquired antilisterial resistance.
FIG. 9.
Involvement of DC-derived IFN-γ in the induction of acquired resistance to a lethal L. monocytogenes infection. IFN-γ-deficient mice were infected intravenously with 5 × 104 CFU of L. monocytogenes, wild-type mice were infected intravenously with 5 × 105 CFU of L. monocytogenes, and DCs were purified from their spleens at 48 h after infection. Wild-type mice were immunized with 5 × 104 DCs from L. monocytogenes-infected, IFN-γ-deficient mice or DCs from L. monocytogenes-infected wild-type mice or uninfected, IFN-γ-deficient mice. Wild-type mice were injected with PBS only as a nonimmune control. They were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. Each result represents the mean ± the standard deviation for a group of four mice. An asterisk indicates a significant difference from the value for the nonimmune control group (P < 0.01). LMDC, L. monocytogenes-infected DCs.
Involvement of DC-derived IL-12 in the induction of host resistance to a lethal L. monocytogenes infection.
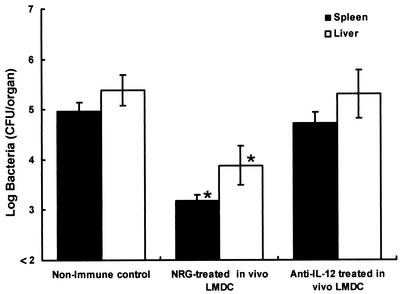
We next investigated whether IL-12 produced by L. monocytogenes-infected DCs is involved in the induction of host resistance to a lethal L. monocytogenes infection. Mice were injected intraperitoneally with 2 mg of an anti-IL-12 p40 MAb or normal rat globulin 2 h before infection with 5 × 105 CFU of L. monocytogenes, and DCs were purified from their spleens at 48 h after infection. Naive mice were immunized with 5 × 104 DCs. Nonimmune control mice were injected with PBS only. Mice of each group were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later, and the numbers of bacteria in their spleens and livers were determined at 48 h postchallenge (Fig. 10). Elimination of bacteria from the organs of mice immunized with in vivo-infected DCs from normal rat globulin-treated mice was significantly higher than that from the organs of nonimmune control mice. In contrast, immunization with in vivo-infected DCs from anti-IL-12 p40 MAb-treated mice produced no enhancement of host resistance to lethal L. monocytogenes infection, suggesting that IL-12 produced by infected DCs may play a critical role in the induction of acquired antilisterial resistance.
FIG. 10.
Involvement of DC-derived IL-12 in the induction of acquired resistance to a lethal L. monocytogenes infection. Mice were given a single injection of 2 mg of an anti-IL-12 p40 MAb or normal rat globulin intravenously 2 h before infection with 5 × 105 CFU of L. monocytogenes, and DCs were purified from their spleens at 48 h after the infection. Naive mice were immunized with 5 × 104 DCs from L. monocytogenes-infected and anti-IL-12 p40 MAb-treated mice or L. monocytogenes-infected and normal rat globulin-treated mice. Nonimmune control mice were injected with PBS only. They were challenged with 5 × 106 CFU of L. monocytogenes 4 weeks later. The numbers of bacteria in the spleens and livers of the mice were determined at 48 h after the challenge. Each result represents the mean ± the standard deviation for a group of four mice. An asterisk indicates a significant difference from the value for the nonimmune control group (P < 0.01). LMDC, L. monocytogenes-infected DCs.
DISCUSSION
Antigen-specific immunity has been successfully induced by immunization with in vitro antigen-pulsed DCs in various systems (4, 15, 18, 20-22, 31, 34, 36). In the present study, we also demonstrated that immunization with in vitro bacterium-pulsed DCs could induce acquired host resistance to L. monocytogenes infection. Moreover, we demonstrated that acquired antilisterial resistance was induced efficiently by immunization with DCs obtained from L. monocytogenes-infected mice in vivo and that IL-12 may play a critical role in induction of resistance.
Mature DCs substantially present antigens to T cells, and their characteristics include up-regulation of major histocompatibility complex class II, CD40, CD54, CD80, and CD86. (17). Immature DCs express an almost undetectable level of CD80 on their surface, while CD86 is expressed persistently on the surfaces of immature peripheral monocytes and DCs (12). Therefore, we investigated the expression of CD80 as an activation marker of DCs. DCs obtained from mice infected with L. monocytogenes for 48 h showed a high level of CD80 expression on their surfaces compared with DCs from naive mice (Fig. 2). In contrast, DCs from mice infected with L. monocytogenes for 6 h showed hardly any up-regulation of CD80 (Fig. 2). Antigen-induced expression of CD80 reportedly peaks after 18 to 42 h in vitro (12). Our present results suggest that enough time may be required for maturation of DCs in vivo.
Previous studies have demonstrated that in vitro antigen-pulsed DCs can induce acquired antigen-specific immunity. DCs pulsed with Mycobacterium bovis bacillus Calmette-Guérin in vitro induced a strong T-cell response to mycobacterial antigens (15), and DCs stimulated with lymphocytic choriomeningitis virus-specific peptides increased cytolytic T-lymphocyte activity (18). DCs pulsed in vitro with viable B. burgdorferi spirochetes mediated a protective response against tick-transmitted spirochetes (22). Our present results demonstrate that DCs pulsed with viable L. monocytogenes in vitro are able to induce acquired antilisterial resistance (Fig. 1). Notably, DCs from mice infected with viable L. monocytogenes also induced host resistance against a lethal infection with L. monocytogenes (Fig. 3). Acquired host resistance to L. monocytogenes induced by immunization with in vivo-infected DCs was antigen specific, since the immunized mice showed no augmented resistance to S. enterica serovar Typhimurium infection (Fig. 4). On the other hand, it is possible that viable L. monocytogenes existing in the DCs used for immunization induced antilisterial resistance. However, antilisterial resistance was not induced by immunization with a dose of viable L. monocytogenes equivalent to that existing in the DCs used for immunization (Fig. 5). It has been reported that mice immunized with HKLM exhibited no protection against an L. monocytogenes challenge (19). In our study, immunization with DCs from HKLM-injected mice failed to induce acquired antilisterial resistance (Fig. 5). Acquisition of antilisterial resistance was dependent on the number of in vivo-infected DCs used for immunization and injection of fewer than 105 DCs failed to induce acquired resistance (Fig. 6). Moreover, when mice were immunized with the same numbers of in vivo-infected DCs or in vitro-pulsed DCs, the number of L. monocytogenes bacteria in the organs of mice immunized with in vivo-infected DCs was about 10-fold lower than that of mice immunized with in vitro-pulsed DCs after a challenge with a lethal dose of L. monocytogenes (Fig. 6), suggesting that in vivo-infected DCs may be able to induce more effective host resistance to a lethal L. monocytogenes infection than in vitro-pulsed DCs.
When DCs capture an antigen, they migrate to peripheral lymphatic tissue and activate naive T cells (2). It has been reported that DCs exposed to Candida yeast cells in vitro activated CD4+ T cells (4) and that Chlamydia-pulsed DCs induced proliferation and IFN-γ production of CD4+ T cells (34). CD8+ T cells are known to play a critical role in elimination of L. monocytogenes from the organs of mice (11). We investigated the capacity of in vivo-infected DCs to stimulate naive T cells (Fig. 7). DCs obtained from L. monocytogenes-infected mice could stimulate proliferation of both T-cell subsets, suggesting that in vivo-infected DCs activate CD8+ T cells, which is critical in acquired antilisterial resistance, as well as CD4+ T cells.
L. monocytogenes infection induces a Th1 response in the host, and IFN-γ plays a critical role in host resistance to L. monocytogenes infection (3, 14). IL-12 is known to induce IFN-γ production by T cells (23, 25). Bone marrow-derived macrophages secrete IFN-γ by IL-12 stimulation (23), and splenic murine DCs and monocytes can produce IFN-γ in response to IL-12 and IL-18 (6), both of which are produced by DCs (33). IL-12 is the key cytokine that drives Th1 differentiation. Our data demonstrate that DCs from L. monocytogenes-infected mice produce higher titers of IFN-γ and IL-12 than do DCs from naive mice (Fig. 8). These data suggest that DCs from mice infected with L. monocytogenes in vivo were activated and had the ability to drive T cells to Th1 cells. We investigated the importance of DC-derived IFN-γ and IL-12 in the induction of acquired antilisterial resistance. When wild-type mice were immunized with DCs from L. monocytogenes-infected IFN-γ-deficient mice, these animals could be protected against a lethal L. monocytogenes challenge (Fig. 9), suggesting that DC-derived IFN-γ may play a marginal role in the induction of acquired resistance. In accordance with the present results, the previous study demonstrated that L. monocytogenes-immunized IFN-γ-deficient mice exhibited host resistance to a secondary lethal challenge with L. monocytogenes (10). Interestingly, although acquired antilisterial resistance was induced in the livers of mice immunized with DCs from IFN-γ-deficient mice, bacterial elimination in their livers was not efficient, compared with that in their spleens (Fig. 9). DCs that have captured an antigen reportedly migrate to lymphatic tissues, such as the lymph nodes and spleen (1, 9), and in vivo-infected DCs may exhibit homing to the lymph nodes and spleen rather than to the liver. Therefore, it is possible that IFN-γ is involved in the migration of injected DCs to the liver. On the other hand, when mice were immunized with DCs from IL-12-neutralized mice, they failed to gain acquired antilisterial resistance (Fig. 10), suggesting that DC-derived IL-12 plays a critical role in the induction of acquired resistance.
In summary, our present study demonstrated that DCs obtained from mice infected with L. monocytogenes in vivo induced acquired resistance to a lethal infection with L. monocytogenes and that IL-12 produced by infected DCs may be involved in the induction of acquired antilisterial resistance. In vivo-pulsed DCs may be useful as a means of inducing antigen-specific immunity or tolerance.
Acknowledgments
This work was supported in part by a grant-in-aid for General Scientific Research (13670261) provided by the Japanese Ministry of Education, Science, Sports and Culture.
Editor: J. D. Clements
REFERENCES
- 1.Austyn, J. M., J. W. Kupiec-Weglinski, D. F. Hankins, and P. J. Morris. 1988. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J. Exp. Med. 167:646-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. R. Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans: implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrick, D. A., M. D. Schrenzel, T. Mulvania, B. Hsieh, W. G. Ferlin, and H. Lepper. 1995. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature 373:255-257. [DOI] [PubMed] [Google Scholar]
- 6.Fukao, T., S. Matsuda, and S. Koyasu. 2000. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-γ production by dendritic cells. J. Immunol. 164:64-71. [DOI] [PubMed] [Google Scholar]
- 7.Grohmann, U., M. L. Belladonna, R. Bianchi, C. Orabona, E. Ayroldi, M. C. Fioretti, and P. Puccetti. 1998. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity 9:315-323. [DOI] [PubMed] [Google Scholar]
- 8.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunn, M. D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L. T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFNγ. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 11.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 12.Hathcock, K. S., G. Laszlo, C. Pucillo, P. Linsley, and R. J. Hodes. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J. Immunol. 166:5448-5455. [DOI] [PubMed] [Google Scholar]
- 14.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-γ receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, K., M. Inaba, M. Naito, and R. M. Steinman. 1993. Dendritic cell progenitors phagocytose particulates, including Bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 17.Kodaira, Y., S. K. Nair, L. E. Wrenshall, E. Gilboa, and J. L. Platt. 2000. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J. Immunol. 165:1599-1604. [DOI] [PubMed] [Google Scholar]
- 18.Ludewig, B., S. Ehl, U. Karrer, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1998. Dendritic cells efficiently induce protective antiviral immunity. J. Virol. 72:3812-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 20.Marriott, I., T. G. Hammond, E. K. Thomas, and K. L. Bost. 1999. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur. J. Immunol. 29:1107-1115. [DOI] [PubMed] [Google Scholar]
- 21.Mayordomo, J. I., T. Zorina, W. J. Storkus, L. Zitvogel, C. Celluzzi, L. D. Falo, C. J. Melief, S. T. Ildstad, W. M. Kast, A. B. Deleo, and M. T. Lotze. 1995. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1:1297-1302. [DOI] [PubMed] [Google Scholar]
- 22.Mbow, M. L., N. Zeidner, N. Panella, R. G. Titus, and J. Piesman. 1997. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect. Immun. 65:3386-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakane, A., S. Nishikawa, S. Sasaki, T. Miura, M. Asano, M. Kohanawa, K. Ishiwata, and T. Minagawa. 1996. Endogenous interleukin-4, but not interleukin-10, is involved in suppression of host resistance against Listeria monocytogenes infection in gamma interferon-depleted mice. Infect. Immun. 64:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon γ production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 189:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roman, M., E. Martin Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 27.Ruedl, C., and M. F. Bachmann. 1999. CTL priming by CD8+ and CD8− dendritic cells in vivo. Eur. J. Immunol. 29:3762-3767. [DOI] [PubMed] [Google Scholar]
- 28.Ruedl, C., C. Rieser, G. Boeck, G. Wick, and H. Wolf. 1996. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer's patches. Eur. J. Immunol. 26:1801-1806. [DOI] [PubMed] [Google Scholar]
- 29.Sato, Y., M. Roman, H. Tighe, D. Lee, M. Corr, M. D. Nguyen, G. J. Silverman, M. Lotz, D. A. Carson, and E. Raz. 1996. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 273:352-354. [DOI] [PubMed] [Google Scholar]
- 30.Shaw, J. H., V. R. Grund, L. Durling, and H. D. Caldwell. 2001. Expression of genes encoding Th1 cell-activating cytokines and lymphoid homing chemokines by chlamydia-pulsed dendritic cells correlates with protective immunizing efficacy. Infect. Immun. 69:4667-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 32.Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, and H. Wagner. 1997. Bacterial DNA causes septic shock. Nature 386:336-337. [DOI] [PubMed] [Google Scholar]
- 33.Stoll, S., H. Jonuleit, E. Schmitt, G. Muller, H. Yamauchi, M. Kurimoto, J. Knop, and A. H. Enk. 1998. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur. J. Immunol. 28:3231-3239. [DOI] [PubMed] [Google Scholar]
- 34.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tagawa, Y., K. Sekikawa, and Y. Iwakura. 1997. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice: role for IFN-γ in activating apoptosis of hepatocytes. J. Immunol. 159:1418-1428. [PubMed] [Google Scholar]
- 36.Worgall, S., T. Kikuchi, R. Singh, K. Martushova, L. Lande, and R. G. Crystal. 2001. Protection against pulmonary infection with Pseudomonas aeruginosa following immunization with P. aeruginosa-pulsed dendritic cells. Infect. Immun. 69:4521-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto, T., H. Sashinami, A. Takaya, T. Tomoyasu, H. Matsui, Y. Kikuchi, T. Hanawa, S. Kamiya, and A. Nakane. 2001. Disruption of the genes for ClpXP protease in Salmonella enterica serovar Typhimurium results in persistent infection in mice, and development of persistence requires endogenous gamma interferon and tumor necrosis factor alpha. Infect. Immun. 69:3164-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]