Abstract
Transglutaminase (TG) activity is increased in the mucosa of patients with coeliac disease. Among 18 patients with untreated coeliac disease we have found a significant decrease (p less than 0.001) in serum levels of TG activity (0.72 (0.23) mU/ml). There was no significant differences between 16 treated coeliacs (1.24 (0.28) mU/ml) and 30 normal controls (1.63 (0.42) mU/ml). To evaluate the connection between serum and mucosal TG activity we used the experimental model of methotrexate induced acute hypoplastic enteropathy in the rat. Transglutaminase activity was unchanged in serum and mucosa 24 and 48 hours after MTX administration, but increased in mucosa (2.606 (0.95) v basal 0.207 (0.026) mU/mg protein, p less than 0.001) and significantly decreased in serum at 72 hours (2.08 (0.38) v basal 5.56 (1.50) mU/ml, p less than 0.001) during intestinal cell proliferation. Activity of the enzyme in the mucosa and serum returned to baseline levels within 120 hours. This experimental animal model helps to explain the data of TG activity in human intestinal mucosa and serum reported in this study. Results are mean (SD).
Full text
PDF

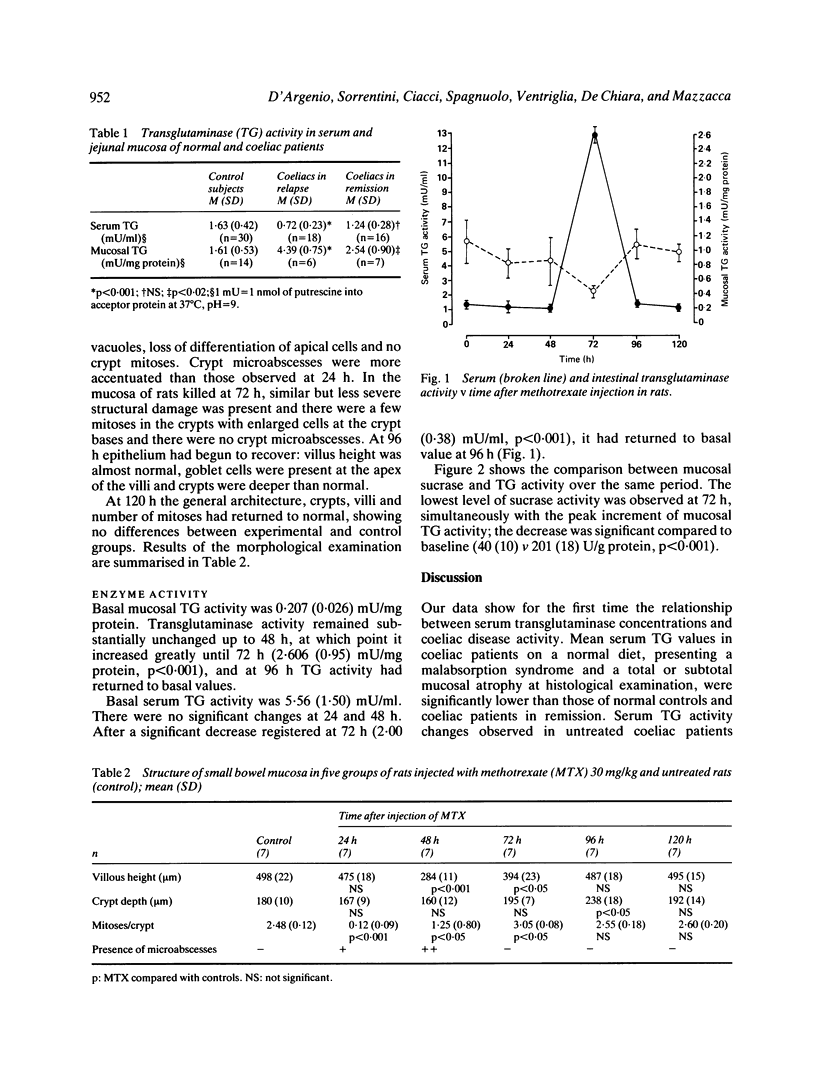
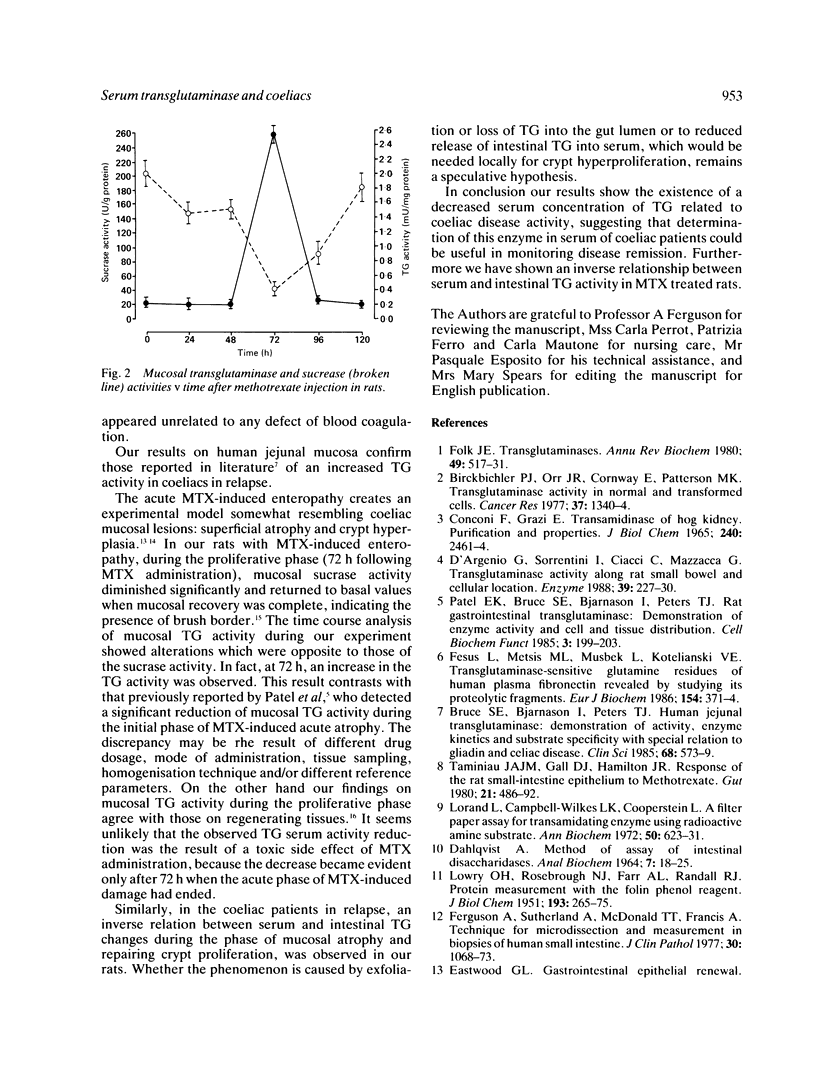

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birckbichler P. J., Orr G. R., Conway E., Patterson M. K., Jr Transglutaminase activity in normal and transformed cells. Cancer Res. 1977 May;37(5):1340–1344. [PubMed] [Google Scholar]
- Bruce S. E., Bjarnason I., Peters T. J. Human jejunal transglutaminase: demonstration of activity, enzyme kinetics and substrate specificity with special relation to gliadin and coeliac disease. Clin Sci (Lond) 1985 May;68(5):573–579. doi: 10.1042/cs0680573. [DOI] [PubMed] [Google Scholar]
- CONCONI F., GRAZI E. TRANSAMIDINASE OF HOG KIDNEY. I. PURIFICATION AND PROPERTIES. J Biol Chem. 1965 Jun;240:2461–2464. [PubMed] [Google Scholar]
- D'Argenio G., Sorrentini I., Ciacci C., Mazzacca G. Transglutaminase activity along the rat small bowel and cellular location. Enzyme. 1988;39(4):227–230. doi: 10.1159/000469123. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Sutherland A., MacDonald T. T., Allan F. Technique for microdissection and measurement in biopsies of human small intestine. J Clin Pathol. 1977 Nov;30(11):1068–1073. doi: 10.1136/jcp.30.11.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesus L., Metsis M. L., Muszbek L., Koteliansky V. E. Transglutaminase-sensitive glutamine residues of human plasma fibronectin revealed by studying its proteolytic fragments. Eur J Biochem. 1986 Jan 15;154(2):371–374. doi: 10.1111/j.1432-1033.1986.tb09407.x. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Increased nuclear conjugated polyamines and transglutaminase during liver regeneration. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1712–1716. doi: 10.1073/pnas.78.3.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Patel E. K., Bruce S. E., Bjarnason I., Peters T. J. Rat gastrointestinal transglutaminase: demonstration of enzyme activity and cell and tissue distributions. Cell Biochem Funct. 1985 Jul;3(3):199–203. doi: 10.1002/cbf.290030307. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Taminiau J. A., Gall D. G., Hamilton J. R. Response of the rat small-intestine epithelium to methotrexate. Gut. 1980 Jun;21(6):486–492. doi: 10.1136/gut.21.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (second of two parts). Mechanisms of control. N Engl J Med. 1978 Jun 29;298(26):1444–1450. doi: 10.1056/NEJM197806292982604. [DOI] [PubMed] [Google Scholar]


