Abstract
Stromal-epithelial interaction is a potent driving force in the developing intestinal mucosa which ensures tissue specific cellular differentiation. The mechanisms involved are relevant to tissue renewal in adult organs yet they have not been elucidated because of the lack of appropriate in vitro models. In this study, we have investigated the interaction between intestinal mesenchymal and epithelial cells at the cellular level in vitro. Fetal rat intestinal epithelial cell colonies explanted in vitro on the 15th day of gestation, which failed to mature in plain monocultures, were reassociated in coculture with three different types of mesenchyme:fetal skin, gastric and intestinal mesenchyme. Only fetal epithelial cells cocultured with intestinal (homologous) mesenchyme acquired definite signs of differentiation within three to six days. These primitive epithelial cells were shown by electronmicroscopy to become highly polarized, connected by tight junctions and covered with a regular brush border. Three brush border enzymes were strongly expressed in homologous cocultures and their activity was sensitive to dexamethasone. In contrast, fetal epithelial cells cocultured with skin or stomach derived mesenchyme under identical conditions failed to differentiate in vitro: they remained flat, unpolarised and expressed only low enzyme activity. The unique potential of the small intestinal mesenchyme to promote intestinal epithelial differentiation is discussed.
Full text
PDF

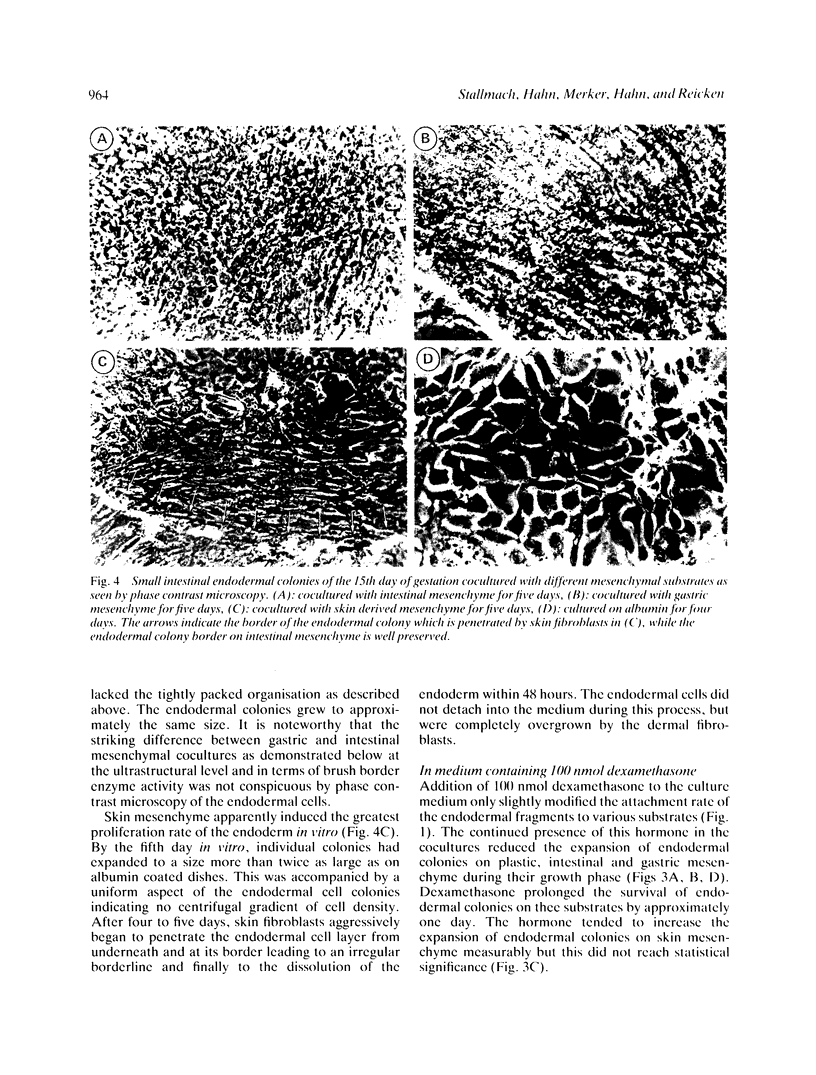
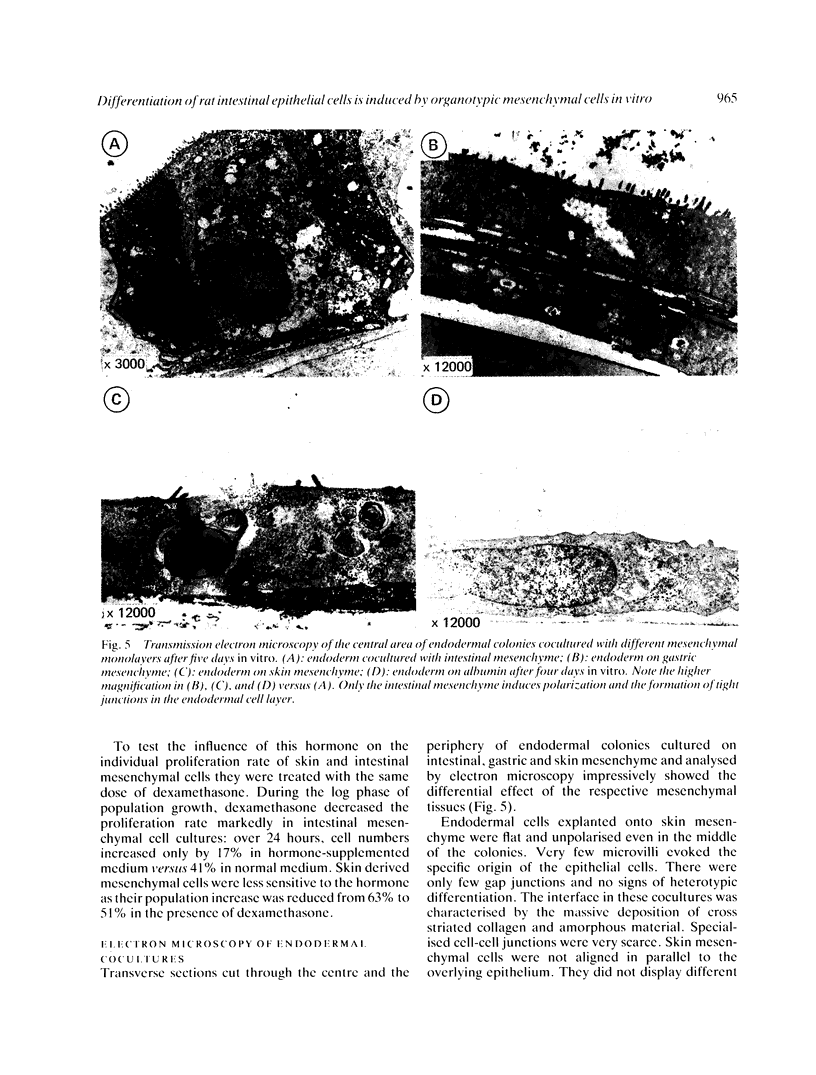





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH R. Morphogenetic interactions in the development of the mouse thymus gland. Dev Biol. 1960 Jun;2:271–284. doi: 10.1016/0012-1606(60)90009-9. [DOI] [PubMed] [Google Scholar]
- Beaulieu J. F., Calvert R. Hormonal regulation of epithelial cell proliferation in the fetal mouse duodenum in vitro. Anat Rec. 1987 Mar;217(3):250–255. doi: 10.1002/ar.1092170305. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Bigsby R. M., Cooke P. S., Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985 Sep;17(3):137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. INTESTINAL INVERTASE: PRECOCIOUS DEVELOPMENT OF ACTIVITY AFTER INJECTION OF HYDROCORTISONE. Science. 1964 Jan 3;143(3601):42–44. doi: 10.1126/science.143.3601.42. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Gutschmidt S., Lange U., Riecken E. O. "In situ"--measurements of protein contents in the brush border region along rat jejunal villi and their correlations with four enzyme activities. Histochemistry. 1981;72(3):467–479. doi: 10.1007/BF00501789. [DOI] [PubMed] [Google Scholar]
- Haffen K., Kedinger M., Simon-Assmann P. Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J Pediatr Gastroenterol Nutr. 1987 Jan-Feb;6(1):14–23. doi: 10.1097/00005176-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Haffen K., Lacroix B., Kedinger M., Simon-Assmann P. M. Inductive properties of fibroblastic cell cultures derived from rat intestinal mucosa on epithelial differentiation. Differentiation. 1983;23(3):226–233. doi: 10.1111/j.1432-0436.1982.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Hahn U. Extracellular matrix proteins in small-intestinal cell cultures. Scand J Gastroenterol Suppl. 1988;151:70–78. doi: 10.3109/00365528809095916. [DOI] [PubMed] [Google Scholar]
- Hahn U., Schuppan D., Hahn E. G., Merker H. J., Riecken E. O. Intestinal cells produce basement membrane proteins in vitro. Gut. 1987;28 (Suppl):143–151. doi: 10.1136/gut.28.suppl.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A., Mizuno T. Intestinal cytodifferentiation in vitro of chick stomach endoderm induced by the duodenal mesenchyme. J Embryol Exp Morphol. 1984 Aug;82:163–176. [PubMed] [Google Scholar]
- Jamieson J. D. Plasmalemmal glycoproteins and basal lamina: involvement in pancreatic morphogenesis. Prog Clin Biol Res. 1982;91:413–427. [PubMed] [Google Scholar]
- Kramer B., Andrew A., Rawdon B. B., Becker P. The effect of pancreatic mesenchyme on the differentiation of endocrine cells from gastric endoderm. Development. 1987 Aug;100(4):661–671. doi: 10.1242/dev.100.4.661. [DOI] [PubMed] [Google Scholar]
- Kédinger M., Simon-Assmann P., Alexandre E., Haffen K. Importance of a fibroblastic support for in vitro differentiation of intestinal endodermal cells and for their response to glucocorticoids. Cell Differ. 1987 Mar;20(2-3):171–182. doi: 10.1016/0045-6039(87)90431-3. [DOI] [PubMed] [Google Scholar]
- Lacroix B., Kedinger M., Simon-Assmann P. M., Haffen K. Effects of human fetal gastroenteric mesenchymal cells on some developmental aspects of animal gut endoderm. Differentiation. 1984;28(2):129–135. doi: 10.1111/j.1432-0436.1984.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Lacroix B., Kedinger M., Simon-Assmann P., Haffen K. Enzymatic response to glucocorticoids of the chick intestinal endoderm associated with various mesenchymal cell types. Biol Cell. 1985;54(3):235–239. doi: 10.1111/j.1768-322x.1985.tb00399.x. [DOI] [PubMed] [Google Scholar]
- MORSON B. C. Precancerous lesions of upper gastrointestinal tract. JAMA. 1962 Feb 3;179:311–315. doi: 10.1001/jama.1962.03050050001001. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Ponniah J., Mathan V. I. Epithelial cell renewal and turnover and relationship to morphologic abnormalities in jejunal mucosa in tropical sprue. Dig Dis Sci. 1986 Jun;31(6):586–592. doi: 10.1007/BF01318689. [DOI] [PubMed] [Google Scholar]
- Mathan M., Hermos J. A., Trier J. S. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol. 1972 Mar;52(3):577–588. doi: 10.1083/jcb.52.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker H. J., Barrach H. J. The morphology of basement membrane formation. Eur J Cell Biol. 1981 Dec;26(1):111–120. [PubMed] [Google Scholar]
- Quaroni A. Development of fetal rat intestine in organ and monolayer culture. J Cell Biol. 1985 May;100(5):1611–1622. doi: 10.1083/jcb.100.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner D. W., Ito S., Rutten M. J., Silen W. A rapid method for culturing guinea pig gastric mucous cell monolayers. In Vitro Cell Dev Biol. 1985 Aug;21(8):453–462. doi: 10.1007/BF02620834. [DOI] [PubMed] [Google Scholar]
- Rothman T. P., Gershon M. D., Fontaine-Pérus J. C., Chanconie M., Le Douarin N. M. The effect of back-transplants of the embryonic gut wall on growth of the neural tube. Dev Biol. 1987 Dec;124(2):331–346. doi: 10.1016/0012-1606(87)90486-6. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P. M., Kedinger M., Grenier J. F., Haffen K. Control of brush border enzymes by dexamethasone in the fetal rat intestine cultured in vitro. J Pediatr Gastroenterol Nutr. 1982;1(2):257–265. doi: 10.1097/00005176-198201020-00017. [DOI] [PubMed] [Google Scholar]
- Smith B. T., Fletcher W. A. Pulmonary epithelial-mesenchymal interactions: beyond organogenesis. Hum Pathol. 1979 May;10(3):248–250. doi: 10.1016/s0046-8177(79)80020-9. [DOI] [PubMed] [Google Scholar]






