Abstract
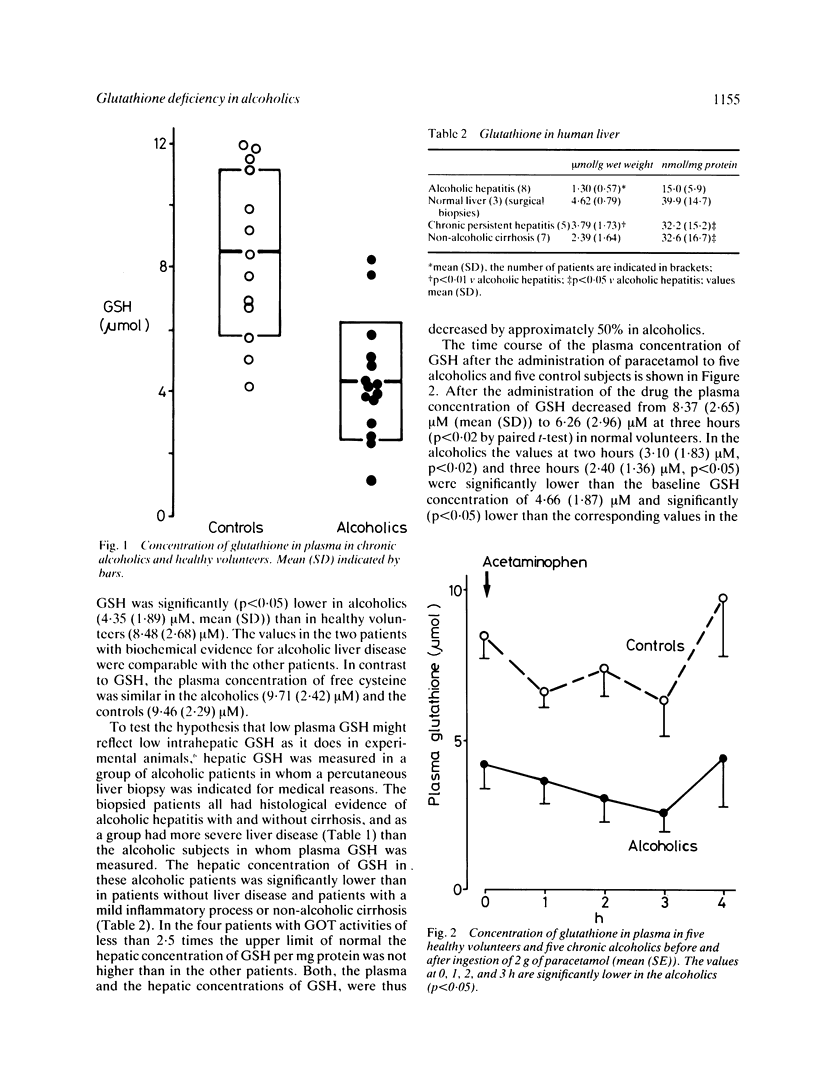
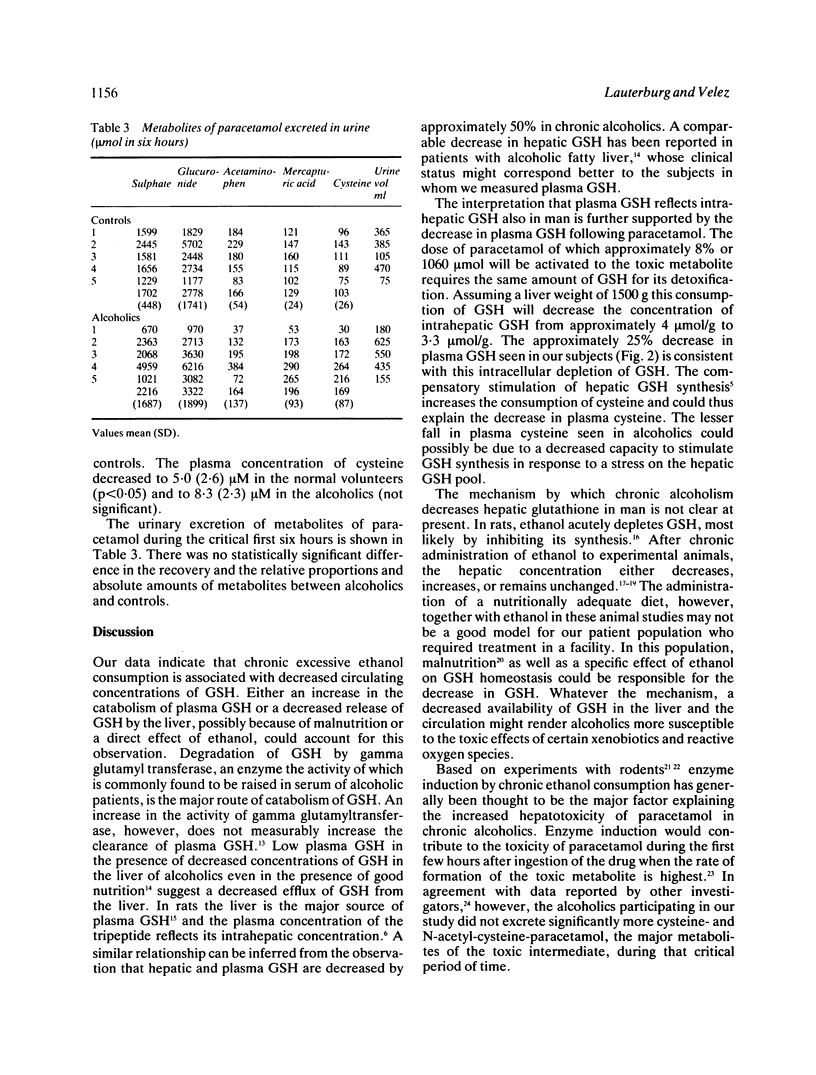
Patients chronically abusing ethanol are more susceptible to the hepatotoxic effects of paracetamol. This could be due to an increased activation of the drug to a toxic metabolite or to a decreased capacity to detoxify the toxic metabolite by conjugation with glutathione (GSH). To test these hypotheses paracetamol 2 g was administered to five chronic alcoholics without clinical evidence of alcoholic liver disease and five control subjects. The urinary excretion of cysteine- plus N-acetyl-cysteine-paracetamol, the two major products of detoxification of the reactive metabolite of paracetamol, was not significantly higher in chronic alcoholics arguing against a substantially increased metabolic activation of paracetamol. Chronic alcoholics had significantly lower plasma concentrations of GSH than healthy volunteers, however (4.35 (1.89) microM v 8.48 (2.68) microM, p less than 0.05) before the administration of paracetamol, and plasma GSH reached lower concentrations in the alcoholics after paracetamol (2.40 (1.36) v 6.26 (2.96) microM). In a group of patients with alcoholic hepatitis intrahepatic GSH was significantly lower than in patients with chronic persistent hepatitis and patients with non-alcoholic cirrhosis, suggesting that low plasma GSH in alcoholics reflects low hepatic concentrations of GSH. The data indicate that low GSH may be a risk factor for paracetamol hepatotoxicity in alcoholics because a lower dose of paracetamol will be necessary to deplete GSH below the critical threshold concentration where hepatocellular necrosis starts to occur.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Lauterburg B. H., Mitchell J. R. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983 Dec;227(3):749–754. [PubMed] [Google Scholar]
- Burgunder J. M., Lauterburg B. H. Decreased production of glutathione in patients with cirrhosis. Eur J Clin Invest. 1987 Oct;17(5):408–414. doi: 10.1111/j.1365-2362.1987.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Guerri C., Grisolia S. Influence of prolonged ethanol intake on the levels and turnover of alcohol and aldehyde dehydrogenases and glutathione. Adv Exp Med Biol. 1980;126:365–384. doi: 10.1007/978-1-4684-3632-7_27. [DOI] [PubMed] [Google Scholar]
- Hétu C., Yelle L., Joly J. G. Influence of ethanol on hepatic glutathione content and on the activity of glutathione S-transferases and epoxide hydrase in the rat. Drug Metab Dispos. 1982 May-Jun;10(3):246–250. [PubMed] [Google Scholar]
- Kater R. M., Roggin G., Tobon F., Zieve P., Iber F. L. Increased rate of clearance of drugs from the circulation of alcoholics. Am J Med Sci. 1969 Jul;258(1):35–39. doi: 10.1097/00000441-196907000-00005. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Adams J. D., Mitchell J. R. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984 Jul-Aug;4(4):586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Davies S., Mitchell J. R. Ethanol suppresses hepatic glutathione synthesis in rats in vivo. J Pharmacol Exp Ther. 1984 Jul;230(1):7–11. [PubMed] [Google Scholar]
- Lauterburg B. H., Mitchell J. R. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol. 1987 Apr;4(2):206–211. doi: 10.1016/s0168-8278(87)80081-8. [DOI] [PubMed] [Google Scholar]
- Licht H., Seeff L. B., Zimmerman H. J. Apparent potentiation of acetaminophen hepatotoxicity by alcohol. Ann Intern Med. 1980 Apr;92(4):511–511. doi: 10.7326/0003-4819-92-4-511_1. [DOI] [PubMed] [Google Scholar]
- McClain C. J., Kromhout J. P., Peterson F. J., Holtzman J. L. Potentiation of acetaminophen hepatotoxicity by alcohol. JAMA. 1980 Jul 18;244(3):251–253. [PubMed] [Google Scholar]
- Mendenhall C. L., Anderson S., Weesner R. E., Goldberg S. J., Crolic K. A. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984 Feb;76(2):211–222. doi: 10.1016/0002-9343(84)90776-9. [DOI] [PubMed] [Google Scholar]
- Misra P. S., Lefévre A., Ishii H., Rubin E., Lieber C. S. Increase of ethanol, meprobamate and pentobarbital metabolism after chronic ethanol administration in man and in rats. Am J Med. 1971 Sep;51(3):346–351. doi: 10.1016/0002-9343(71)90270-1. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Morton S., Mitchell M. C. Effects of chronic ethanol feeding on glutathione turnover in the rat. Biochem Pharmacol. 1985 May 1;34(9):1559–1563. doi: 10.1016/0006-2952(85)90699-9. [DOI] [PubMed] [Google Scholar]
- Newton G. L., Dorian R., Fahey R. C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson F. J., Holloway D. E., Erickson R. R., Duquette P. H., McClain C. J., Holtzman J. L. Ethanol induction of acetaminophen toxicity and metabolism. Life Sci. 1980 Nov 3;27(18):1705–1711. doi: 10.1016/0024-3205(80)90646-3. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Critchley J. A. Drug interactions affecting analgesic toxicity. Am J Med. 1983 Nov 14;75(5A):113–116. doi: 10.1016/0002-9343(83)90241-3. [DOI] [PubMed] [Google Scholar]
- Sato C., Matsuda Y., Lieber C. S. Increased hepatotoxicity of acetaminophen after chronic ethanol consumption in the rat. Gastroenterology. 1981 Jan;80(1):140–148. [PubMed] [Google Scholar]
- Shaw S., Rubin K. P., Lieber C. S. Depressed hepatic glutathione and increased diene conjugates in alcoholic liver disease. Evidence of lipid peroxidation. Dig Dis Sci. 1983 Jul;28(7):585–589. doi: 10.1007/BF01299917. [DOI] [PubMed] [Google Scholar]
- Slattery J. T., Wilson J. M., Kalhorn T. F., Nelson S. D. Dose-dependent pharmacokinetics of acetaminophen: evidence of glutathione depletion in humans. Clin Pharmacol Ther. 1987 Apr;41(4):413–418. doi: 10.1038/clpt.1987.50. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]


