Abstract
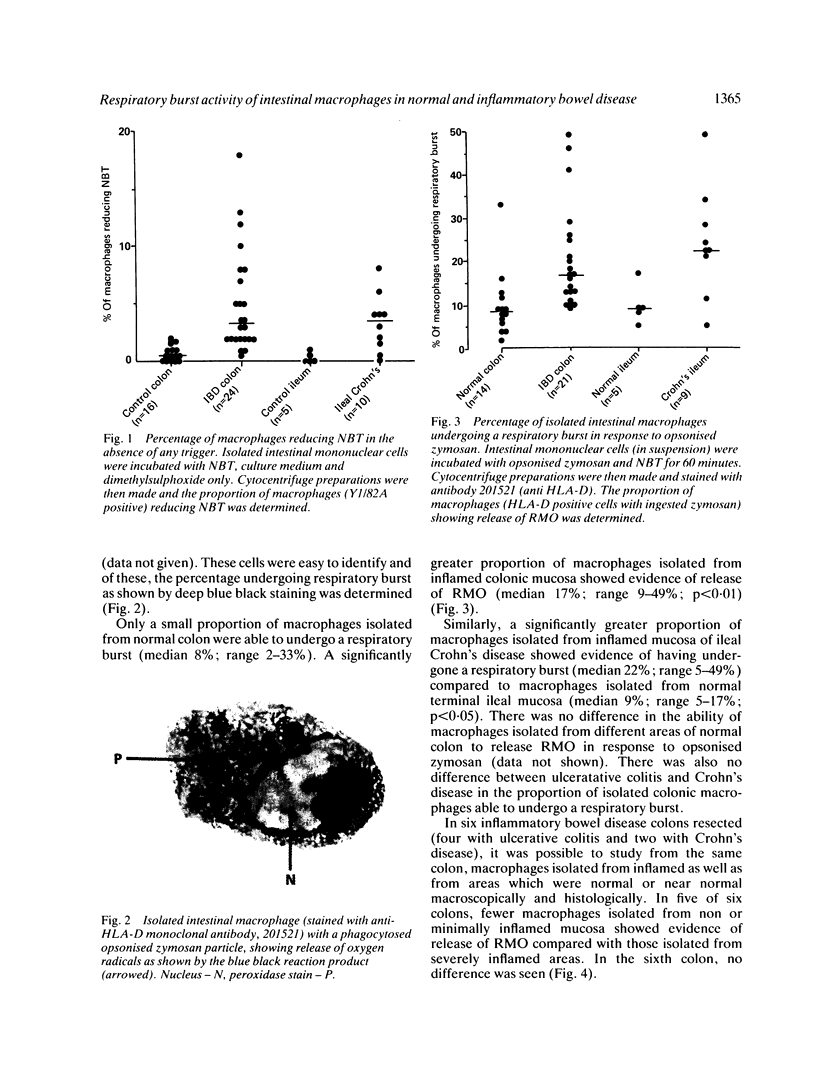
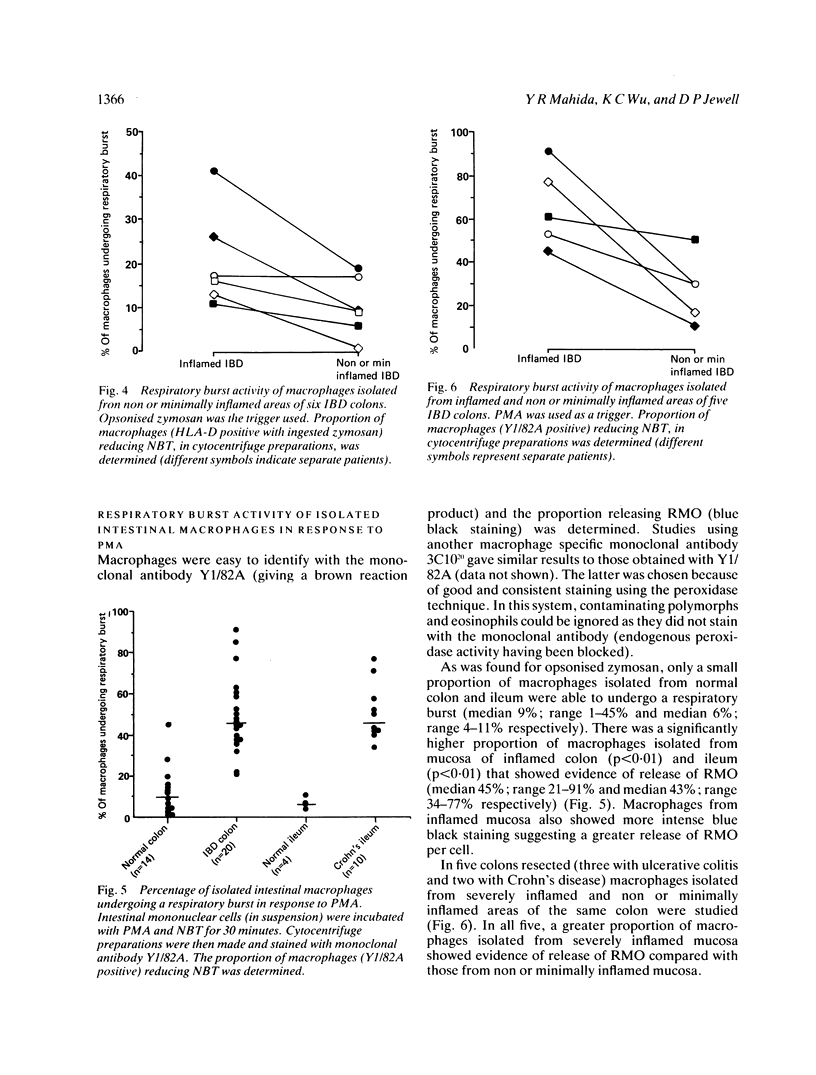
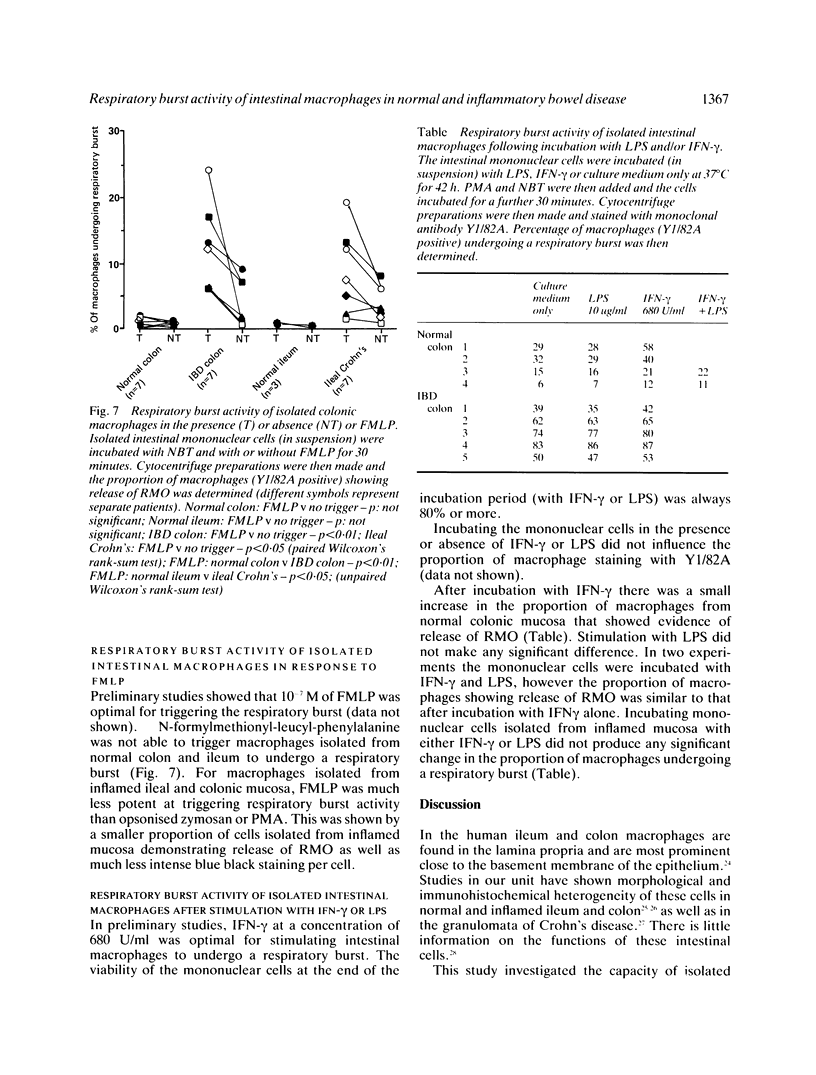
Macrophages isolated from normal mucosa (greater than 5 cm from tumour) and inflamed mucosa (from patients with inflammatory bowel disease) of colon and ileum were studied for their ability to undergo a respiratory burst as assessed by reduction of nitroblue tetrazolium to formazan. Using phorbol myristate acetate (PMA) and opsonised zymosan as triggers, only a minority (median: 8% for zymosan and 9% for PMA) of macrophages isolated from normal colonic mucosa demonstrated release of oxygen radicals. In contrast, a significantly greater (median: 17% for zymosan and 45% for PMA) proportion of macrophages isolated from inflamed colonic mucosa were able to undergo respiratory burst. Studies with normal and inflamed ileum showed similar results. Stimulation of macrophages isolated from normal colon with interferon-gamma produced only a small increase in the proportion of cells showing release of oxygen radicals. We conclude that the respiratory burst capacity of majority of macrophages isolated from normal colon and ileum is downregulated and a greater proportion of macrophages isolated from inflamed colon and ileum are able to undergo a respiratory burst.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Beeken W., Mieremet-Ooms M., Ginsel L. A., Leijh P. C., Verspaget H. Enrichment of macrophages in cell suspensions of human intestinal mucosa by elutriation centrifugation. J Immunol Methods. 1984 Oct 12;73(1):189–201. doi: 10.1016/0022-1759(84)90044-9. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983 Aug;49(4):693–704. [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Davies P. Possible autoregulatory functions of the secretory products of mononuclear phagocytes. Contemp Top Immunobiol. 1984;13:199–223. doi: 10.1007/978-1-4757-1445-6_10. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey F. R., Cordell J. L., Erber W. N., Pulford K. A., Gatter K. C., Mason D. Y. Monoclonal antibody (Y1/82A) with specificity towards peripheral blood monocytes and tissue macrophages. J Clin Pathol. 1988 Jul;41(7):753–758. doi: 10.1136/jcp.41.7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol. 1987 Sep 15;139(6):1971–1977. [PubMed] [Google Scholar]
- Doe W. F., Dorsman B. Chronic inflammatory bowel disease--increased plasminogen activator secretion by mononuclear phagocytes. Clin Exp Immunol. 1982 Apr;48(1):256–260. [PMC free article] [PubMed] [Google Scholar]
- Donnellan W. L. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterology. 1965 Nov;49(5):496–514. [PubMed] [Google Scholar]
- Ezekowitz R. A., Gordon S. Down-regulation of mannosyl receptor-mediated endocytosis and antigen F4/80 in bacillus Calmette-Guérin-activated mouse macrophages. Role of T lymphocytes and lymphokines. J Exp Med. 1982 Jun 1;155(6):1623–1637. doi: 10.1084/jem.155.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Orkin S. H., Newburger P. E. Recombinant interferon gamma augments phagocyte superoxide production and X-chronic granulomatous disease gene expression in X-linked variant chronic granulomatous disease. J Clin Invest. 1987 Oct;80(4):1009–1016. doi: 10.1172/JCI113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., MacPherson G. G., Gordon S. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro. Role of type 3 complement receptors and macrophage-derived complement. J Clin Invest. 1985 Dec;76(6):2368–2376. doi: 10.1172/JCI112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fligiel S. E., Lee E. C., McCoy J. P., Johnson K. J., Varani J. Protein degradation following treatment with hydrogen peroxide. Am J Pathol. 1984 Jun;115(3):418–425. [PMC free article] [PubMed] [Google Scholar]
- Golder J. P., Doe W. F. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983 Apr;84(4):795–802. [PubMed] [Google Scholar]
- Harley A., Starmer C. F., Greenfield J. C., Jr Pressure-flow studies in man. An evaluation of the duration of the phases of systole. J Clin Invest. 1969 May;48(5):895–905. doi: 10.1172/JCI106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson C. H., Butt T. J., Ferry D. M., Hunter J., Chadwick V. S., Broom M. F. Enterohepatic circulation of bacterial chemotactic peptide in rats with experimental colitis. Gastroenterology. 1988 Apr;94(4):1006–1013. doi: 10.1016/0016-5085(88)90560-4. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Pizzo S. V., Imber M. J., Adams D. O. Receptors for maleylated proteins regulate secretion of neutral proteases by murine macrophages. Science. 1982 Nov 5;218(4572):574–576. doi: 10.1126/science.6289443. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Gionchetti P., Vaux D., Jewell D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989 Jun;30(6):826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Mee A. S., Szawatakowski M., Jewell D. P. Monocytes in inflammatory bowel disease: phagocytosis and intracellular killing. J Clin Pathol. 1980 Oct;33(10):921–925. doi: 10.1136/jcp.33.10.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret G., Bitzi A., Hammer B. Macrophage turnover in Crohn's disease and ulcerative colitis. Gastroenterology. 1978 Mar;74(3):501–503. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Poulter L. W., Hobbs S., Jewell D. P., Janossy G. Heterogeneity of HLA-DR-positive histiocytes in human intestinal lamina propria: a combined histochemical and immunohistological analysis. J Clin Pathol. 1983 Apr;36(4):379–384. doi: 10.1136/jcp.36.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. G., Austyn J. M., Hariri G., Beverley P. C., Morris P. J. T cell activation by anti-T3 antibodies: comparison of IgG1 and IgG2b switch variants and direct evidence for accessory function of macrophage Fc receptors. Eur J Immunol. 1986 May;16(5):478–486. doi: 10.1002/eji.1830160503. [DOI] [PubMed] [Google Scholar]
- Strober W., James S. P. The immunologic basis of inflammatory bowel disease. J Clin Immunol. 1986 Nov;6(6):415–432. doi: 10.1007/BF00915248. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspaget H., Beeken W. Mononuclear phagocytes in the gastrointestinal tract. Acta Chir Scand Suppl. 1985;525:113–126. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]