Abstract
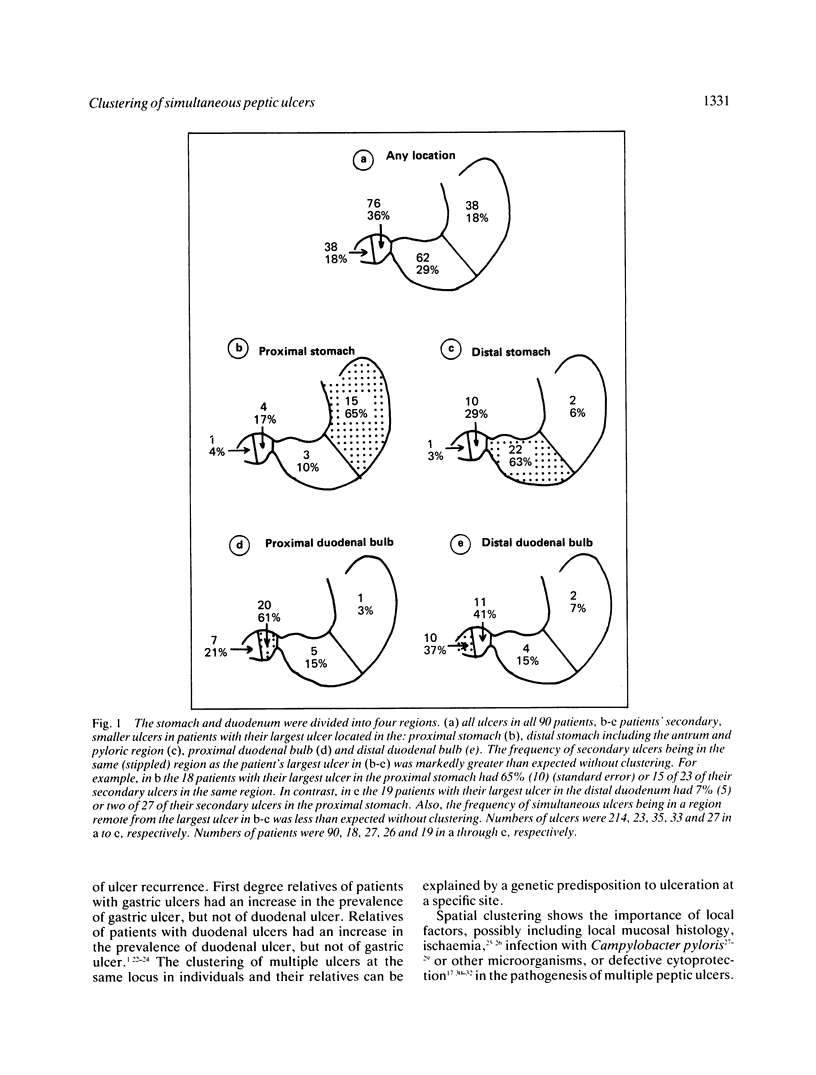
In an endoscopic study of 90 consecutive patients with more than one peptic ulcer, the ulcers in an individual were profoundly spatially clustered. Clustering of ulcer locations was shown using a non-parametric test of clustering (Kruskal-Wallis statistic with 89 degrees of freedom = 151.31, probability of observing this extreme statistic with no clustering less than 0.0005) and a parametric test of clustering (F test statistic with 89 and 124 degrees of freedom = 5.41, probability of observing this extreme statistic with no clustering less than 0.0005). Patients having their largest ulcer in any given region had a much greater likelihood than other patients of having other ulcers in that same site. For example, the 26 patients with their largest ulcer in the proximal duodenal bulb had 20 of 33, or 61% (9) (standard error), of their other ulcers in the proximal duodenal bulb. In contrast, the 18 patients with their largest ulcer in the proximal stomach had four of 23, or 17% (8), of their other ulcers in the proximal duodenal bulb. Of the 59 patients who had two simultaneous ulcers, 28 patients had adjacent ulcers (distance between ulcers less than 4% of the distance from the gastric cardia to the apex of the duodenal bulb). These findings suggest that local factors may be important in the pathogenesis of simultaneous peptic ulcers, including infection caused by Campylobacter pylori or other microorganisms, ischaemia and mucosal barrier disruption.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987 Aug;93(2):371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- Bodemar G., Walan A., Lundquist G. Food-stimulated acid secretion measured by intragastric titration with bicarbonate in patients with duodenal and gastric ulcer disease and in controls. Scand J Gastroenterol. 1978;13(8):911–918. doi: 10.3109/00365527809181368. [DOI] [PubMed] [Google Scholar]
- DOLL R., KELLOCK T. D. The separate inheritance of gastric and duodenal ulcers. Ann Eugen. 1951 Dec;16(3):231–240. doi: 10.1111/j.1469-1809.1951.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Dagradi A. E., Falkner R. E., Lee E. R. Multiple benign gastric ulcers. A clinical and gastroscopic study. Am J Gastroenterol. 1974 Jul;62(1):36–45. [PubMed] [Google Scholar]
- Hsu T. C. Traumatic perforation of ileal pouch. Report of a case. Dis Colon Rectum. 1989 Jan;32(1):64–66. doi: 10.1007/BF02554728. [DOI] [PubMed] [Google Scholar]
- Hui W. M., Lam S. K. Multiple duodenal ulcer: natural history and pathophysiology. Gut. 1987 Sep;28(9):1134–1141. doi: 10.1136/gut.28.9.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. D. Gastric ulcer: classification, blood group characteristics, secretion patterns and pathogenesis. Ann Surg. 1965 Dec;162(6):996–1004. doi: 10.1097/00000658-196512000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R. M. Are gastric and duodenal ulcers separate diseases or do they form a continuum? Dig Dis Sci. 1981 Feb;26(2):149–154. doi: 10.1007/BF01312235. [DOI] [PubMed] [Google Scholar]
- Lam S. K., Lai C. L. Gastric ulcers with and without associated duodenal ulcer have different pathophysiology. Clin Sci Mol Med. 1978 Jul;55(1):97–102. doi: 10.1042/cs0550097. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R., Longstreth G. F., Deering T. B., Summerskill W. H., Go V. L. Gastric secretion and emptying after ordinary meals in duodenal ulcer. Gastroenterology. 1977 Nov;73(5):989–994. [PubMed] [Google Scholar]
- McCray R. S., Ferris E. J., Herskovic T., Winawer S. J., Shapiro J. H., Zamcheck N. Clinical differences between gastric ulcers with and without duodenal deformity. Ann Surg. 1968 Nov;168(5):821–823. doi: 10.1097/00000658-196811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin J. C., Green W. E., Buchanan K. D. Gastric emptying of ingested acid and its effects on plasma gastrin and secretin in duodenal ulcer subjects. Scand J Gastroenterol. 1978;13(3):313–319. doi: 10.3109/00365527809179826. [DOI] [PubMed] [Google Scholar]
- Pihan G., Majzoubi D., Haudenschild C., Trier J. S., Szabo S. Early microcirculatory stasis in acute gastric mucosal injury in the rat and prevention by 16,16-dimethyl prostaglandin E2 or sodium thiosulfate. Gastroenterology. 1986 Dec;91(6):1415–1426. doi: 10.1016/0016-5085(86)90195-2. [DOI] [PubMed] [Google Scholar]
- Price A. B., Levi J., Dolby J. M., Dunscombe P. L., Smith A., Clark J., Stephenson M. L. Campylobacter pyloridis in peptic ulcer disease: microbiology, pathology, and scanning electron microscopy. Gut. 1985 Nov;26(11):1183–1188. doi: 10.1136/gut.26.11.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R. Leg ulceration in sickle cell anemia. Arch Intern Med. 1974 Apr;133(4):690–694. [PubMed] [Google Scholar]
- Szabo S., Trier J. S., Brown A., Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985 Jan;88(1 Pt 2):228–236. doi: 10.1016/s0016-5085(85)80176-1. [DOI] [PubMed] [Google Scholar]
- Tarpila S., Samloff I. M., Pikkarainen P., Vuoristo M., Ihamäki T. Endoscopic and clinical findings in first-degree relative of duodenal ulcer patients and control subjects. Scand J Gastroenterol. 1982 Jun;17(4):503–506. doi: 10.3109/00365528209182239. [DOI] [PubMed] [Google Scholar]
- Veselý K. T., Kubícková Z., Dvoráková M. Clinical data and characteristics differentiating types of peptic ulcer. Gut. 1968 Feb;9(1):57–68. doi: 10.1136/gut.9.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan F., Pearson J., Allen A., Venables C. Changes in the structure of the mucous gel on the mucosal surface of the stomach in association with peptic ulcer disease. Gastroenterology. 1982 May;82(5 Pt 1):827–831. [PubMed] [Google Scholar]


