Hydrogen peroxide is a major reactive oxygen species (ROS) in living organisms, and its homeostasis can have diverse physiological and pathological consequences.1 H2O2 is a source of oxidative stress,2 and oxidative damage resulting from cellular imbalance of H2O2 and other ROS oxidants is connected to aging and severe human diseases such as cancer,3 cardiovascular disorders,4 and Alzheimer's and related neurodegenerative diseases.5 On the other hand, emerging evidence supports a physiological role for H2O2 as a second messenger in cellular signal transduction.6-8 For example, peroxide bursts trigger mitogen-activated protein (MAP) kinase9 and nuclear factor κB (NF-κB)10 pathways that affect cell proliferation and cell death.
Despite the importance of H2O2 to human health and disease, the molecular mechanisms of its production, accumulation, trafficking, and function are insufficiently understood even in the simplest eukaryotic organisms.2 We are interested in developing new chemical tools to study the physiological and pathological roles of H2O2 and related ROS in living systems. In this regard, fluorescent probes are well suited to meet the need for reagents to interrogate the cellular chemistry of H2O2 at the molecular level. One major challenge to achieving this goal is creating water-soluble systems that report H2O2 selectively over competing cellular ROS like superoxide (O2−), nitric oxide (NO), and lipid alkylperoxides. Synthetic small molecules offer one approach to such probes, and several types of reagents have been examined for H2O2 detection. Included are dihydro analogs of fluorescent dyes (e.g., 2′,7′-dichlorodihydrofluorescein (DCFH), Amplex Red, dihydrorhodamine 123),11-13 phosphine-derivatized fluorophores,14,15 lanthanide coordination complexes,16 and chromophores with ROS-cleavable protecting groups.17-19 However, limitations of currently available H2O2-responsive probes include interfering background fluorescence from other ROS, the need for an external activating enzyme, lack of water solubility or compatibility, and/or excitation profiles in the ultraviolet region, which can damage living samples and cause interfering autofluorescence from native cellular species. The most commonly used fluorophore for cellular ROS detection, DCFH, is also easily autoxidized and exhibits increased background fluorescence upon continued exposure to light.17 In this report, we present the synthesis and properties of Peroxyfluor-1 (PF1, 2), a new water-soluble, turn-on optical probe for H2O2 that exhibits high selectivity and dynamic range for this small molecule over other ROS, and establish its utility for imaging changes in [H2O2] within living mammalian cells with visible wavelength excitation and emission energies.
Our strategy for the optical detection of H2O2 relies on the selective H2O2-mediated transformation of arylboronates to phenols.20 In particular, we reasoned that installation of boronic ester groups at the 3′ and 6′ positions of a xanthenone scaffold would force this platform to adopt a closed, colorless, and non-fluorescent lactone form. Upon treatment with H2O2, hydrolytic deprotection of the boronates would subsequently generate the open, colored, and fluorescent fluorescein product. Scheme 1 outlines the preparation of PF1 based on this design. Acid-catalyzed condensation of 3-iodophenol and phthalic anhydride affords 3′,6′-diiodofluoran 1 in 25% yield.21 Palladium-catalyzed transmetallation of fluoran 1 under Masuda conditions with bis(pinacolato)diboron proceeds smoothly to generate PF1 in 50% yield after workup and purification by column chromatography.
Scheme 1.
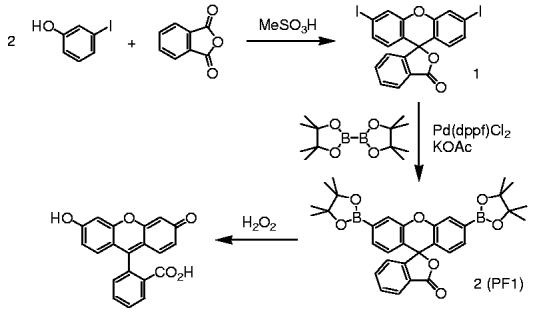
Synthesis of Peroxyfluor-1 (PF1).
PF1 was evaluated under simulated physiological conditions (20 mM HEPES buffer, pH 7). As expected, the parent compound is non-fluorescent and displays no absorption features in the visible region. The addition of H2O2 triggers a prompt fluorescence increase (Figure 1A) with concomitant growth of a visible wavelength absorption band characteristic of fluorescein. Absorption and emission spectra, along with electrospray ionization mass spectrometry, confirm that fluorescein is the product generated from the reaction between PF1 and H2O2. The dynamic range of this probe is large owing to its binary absorption/emission response. The fluorescence response of PF1 is also highly H2O2 selective. Figure 1B compares the relative reactivities of PF1 toward various ROS. PF1 exhibits a greater than 500-fold higher response for H2O2 over similar ROS such as tert-butyl hydroperoxide (TBHP), O2−, NO, or −OCl, which represents a 10 to 100-fold increase in H2O2 selectivity compared to previously reported probes.11,18,19 The xanthenone probe is also more responsive to H2O2 compared to highly reactive oxygen radicals such as •OtBu (> 15-fold higher for H2O2) and •OH (> 3-fold higher for H2O2). We suggest that the observed selectivity for H2O2 over more oxidizing ROS is based on the detection mechanism of PF1, which relies on deprotection rather than oxidation to provide an optical response.
Figure 1.
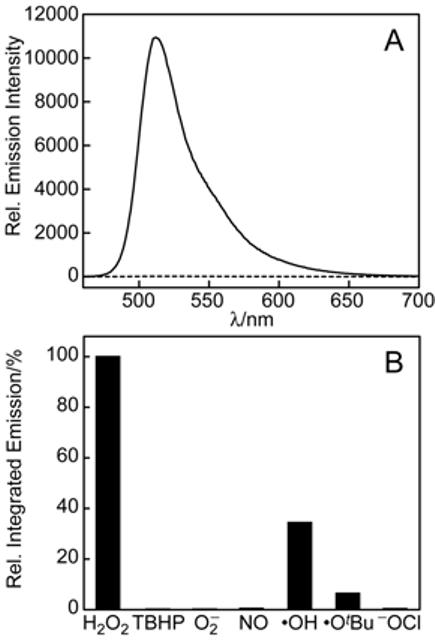
(A) Fluorescence response of 5 μM PF1 to 100 μM H2O2. The dotted and solid line spectra were recorded before and after H2O2 addition, respectively. Spectra were acquired in 20 mM HEPES, pH 7 (λexc = 450 nm). (B) Fluorescence responses of 5 μM PF1 to various ROS (10 mM O2−, 100 μM for all other ROS). •OH and •OtBu were generated by reaction of Fe2+ with H2O2 or TBHP, respectively. NO was delivered using S-nitrosocysteine (SNOC). Spectra were acquired in 20 mM HEPES, pH 7, and all data were obtained after incubation with the appropriate ROS at 25 °C for 1 h. Collected emission was integrated between 460 and 700 nm (λexc = 450 nm).
We next assessed the ability of PF1 to operate within living cells. HEK cells were incubated with 5 μM PF1 for 5 min at 25 °C and show negligible intracellular background fluorescence (Figure 2A). Prompt increases in cytosolic fluorescence are observed upon addition of physiologically relevant concentrations of exogenous H2O2 (10-100 μM, Figure 2B) as determined from scanning confocal microscopy on live samples. Control experiments performed without dye or H2O2 give negligible fluorescence responses, and transmittance measurements after PF1 incubation and H2O2 addition (Figure 2C) confirm that the cells are viable throughout the imaging experiments. These data establish that PF1 is membrane-permeable and can respond to μM changes in H2O2 concentrations within living cells. In addition, subsequent experiments show that fluorescence responses down to 100 nM H2O2 are readily detectible in vitro.
Figure 2.
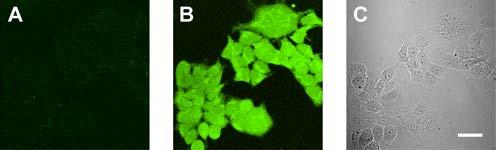
Confocal fluorescence and phase contrast images of live HEK cells. (A) Fluorescence image of HEK cells incubated with 5 μM PF1 for 5 min at 25 °C. (B) Fluorescence image of PF1-stained HEK cells treated with 100 μM H2O2 for 5 min at 25 °C. (C) Brightfield image of live HEK cells after H2O2 addition to confirm viability. Scale bar = 30 μm.
To close, we have presented the synthesis, properties, and biological applications of PF1, a new type of probe for optical imaging of intracellular H2O2. This fluorescein-based reagent features excellent selectivity for H2O2 over competing cellular ROS, large dynamic response range owing to its dual colorimetric/fluorometric detection mechanism, and long-wavelength visible excitation and emission profiles to minimize cell and tissue damage while avoiding interfering autofluorescence from native cellular species. Furthermore, we have demonstrated the value of this probe by measuring changes in intracellular [H2O2] within living mammalian cells. Current efforts are directed toward applying PF1 and related tools for studying the oxidation biology of living systems.
Supplementary Material
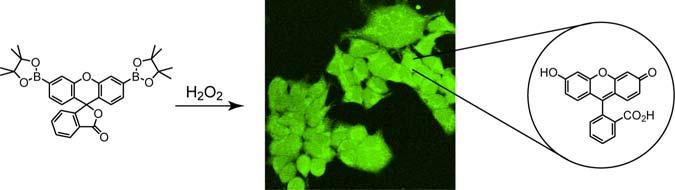
We present the synthesis, properties, and biological applications of Peroxyfluor-1 (PF1), a new type of optical probe for intracellular imaging of hydrogen peroxide in living biological samples. PF1 utilizes a boronate deprotection mechanism to provide unprecedented selectivity and optical dynamic range for detecting H2O2 in aqueous solution over similar reactive oxygen species including superoxide, nitric oxide, tert-butyl hydroperoxide, and hydroxyl radical. We further demonstrate the value of this reagent for biological applications by imaging changes in [H2O2] in living mammalian cells.
Acknowledgment
We thank the University of California, Berkeley for startup funds and the Camille and Henry Dreyfus Foundation for a New Faculty Award.
Footnotes
Supporting Information Available: Synthetic and experimental details (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd Clarendon Press; Oxford, UK: 1999. [Google Scholar]
- 2.Finkel T. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Ohshima H, Tatemichi M, Sawa T. Arch. Biochem. Biophys. 2003;417:3–11. doi: 10.1016/s0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- 4.Shah AM, Channon KM. Heart. 2004;90:486–487. doi: 10.1136/hrt.2003.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnham KJ, Masters CL, Bush AI. Nature Rev. Drug Disc. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 6.Wood ZA, Poole LB, Karplus PA. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 7.Woo HA, Chae HZ, Hwang SC, Yang K-S, Kang SW, Kim K, Rhee SG. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 8.Budanov AV, Slabina AA, Feinstein E, Koonin EV, Chumakov PM. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 9.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. J. Biol. Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. Chem. Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 11.Negre-Salvayre A, Augé N, Duval C, Robbesyn F, Thiers J-C, Nazzal D, Benoist H, Salvayre R. Meth. Enzymol. 2002;352:62–71. doi: 10.1016/s0076-6879(02)52007-3. [DOI] [PubMed] [Google Scholar]
- 12.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Free Rad. Biol. Med. 1999;27:146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 13.Haugland RP. Handbook of Fluorescent Probes and Research Products. 9th Molecular Probes; Eugene, OR: 2002. [Google Scholar]
- 14.Akasaka K, Suzuki T, Ohrui H, Meguro H. Anal. Lett. 1987;20:731–745. [Google Scholar]
- 15.Onoda M, Uchiyama S, Endo A, Tokuyama H, Santa T, Imai K. Org. Lett. 2003;5:1459–1461. doi: 10.1021/ol0342150. [DOI] [PubMed] [Google Scholar]
- 16.Wolfbeis OS, Dürkop A, Wu M, Lin Z. Angew. Chem. Int. Ed. 2002;41:4495–4498. doi: 10.1002/1521-3773(20021202)41:23<4495::AID-ANIE4495>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. J. Biol. Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 18.Lo L-C, Chu C-Y. Chem. Commun. 2003:2728–2729. doi: 10.1039/b309393j. [DOI] [PubMed] [Google Scholar]
- 19.Maeda H, Fukuyasu Y, Yoshida S, Fukuda M, Saeki K, Matsuno H, Yamauchi Y, Yoshida K, Hirata K, Miyamoto K. Angew. Chem. Int. Ed. 2004;43:2389–2391. doi: 10.1002/anie.200452381. [DOI] [PubMed] [Google Scholar]
- 20.Kuivila HG, Armour AG. J. Am. Chem. Soc. 1957;79:5659–5662. [Google Scholar]
- 21.Gronowska J, Dabkowska-Naskret H. Pol. J. Chem. 1981;55:2151–2163. The corresponding 3′,6′-difluoro, 3′,6′-dichloro, and 3′,6′-dibromofluoran analogues have been reported previously. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


