Abstract
Reduced fasting or postprandial gastric volumes have been implicated in the pathophysiology of functional dyspepsia. The mechanisms that underlie the control of gastric fundic volume are incompletely understood, partly because of an inability to accurately measure fundic volume in vivo in small animals. Small animals are useful models to evaluate mechanisms, e.g. in knockout animals. The aim of this study was to determine if an ultrasonometric technique accurately monitors fundic contraction and relaxation in mice in vivo and to determine the effect of modulation of cholinergic, nitrergic and serotonergic pathways on fundic size and compliance in the intact mouse innervated stomach. Two to 4 piezo-electric crystals (diameter 1 mm, 24 μm resolution) were glued to the serosal side of fundus and used to measure distance. Validation studies showed excellent correlation between measured changes and actual changes in distances between crystals and excellent reproducibility. The expected responses to pharmacological modulation with bethanechol and nitroglycerin were demonstrated. Atropine increased the distance between the crystals suggesting a baseline cholinergic regulation of fundic volume. Bethanechol, L-NNA and the 5-HT1B/D agonist sumatriptan decreased the distance between the crystals suggesting fundic contraction. Atropine, nitroglycerin and buspirone caused an increase in intercrystal distance consistent with fundic relaxation. Fundic compliance was investigated by changing intragastric pressure via an implanted catheter. Sumatriptan increased compliance, while buspirone increased the distance between crystals but did not change compliance. The data suggest that ultrasonomicrometry is a useful tool that can reproducibly and accurately measure changes in fundic size and the response to pharmacological agents.
Keywords: ultrasound, smooth muscle, gastric fundus, serotonin
Introduction
The three main motor functions of the human stomach are to accommode food ingested at mealtimes, triturate food and mix the content with digestive enzymes and acid. Gastric accommodation includes receptive relaxation and adaptive relaxation. Receptive relaxation allows the stomach to initially accommodate the volume of a meal without a rise in intragastric pressure (9, 21), whereas adaptive relaxation is a slower response that may be modulated by the physical and chemical properties of the meal ingested (13, 25), and the neurohormonal responses to the meal.
Impaired fundic accommodation can occur as a result of vagal injury or a vagotomy, and is also present in about a third of patients with functional dyspepsia.(3, 34, 36, 37). Fundic accommodation requires an intact extrinsic innervation, predominantly but not exclusively, through vago-vagal reflexes, and a complex interaction between enteric nerves, gastric mucosa, muscularis propria, smooth muscle cells and interstitial cells of Cajal (4, 35, 39). In several species, including humans, the neurotransmitters involved include nitric oxide (NO) and serotonin (5HT) (18, 19, 32, 40). An intact nitrergic pathway is also required for fundic relaxation in the isolated murine fundus (11, 14, 28, 31, 33). However, the mouse fundic data were obtained in muscle strips. The 5HT receptor agonists buspirone and sumatriptan have been used in clinical studies (7, 16) to treat functional dyspepsia by targeting fundic relaxation. Buspirone is a 5-HT1A agonist and sumatriptan a 5HT1B/D agonist, although it is also clear that both act on more than one 5HT receptor subtype (24). The relative contributions of central and peripheral mechanisms of action of both drugs have not been well defined. Research on the neuronal and non-neuronal pathways that lead to fundic accommodation is hampered by the lack of an accurate, reliable and reproducible method to study fundic accommodation in small animals in vivo. Ultrasonography, single photon emission computed tomography (SPECT) and the barostat (13) are used in vivo in larger animals, including humans, but their utility is severely limited in smaller animals such as mice. A recent study developed a miniaturized method to assess gastric tone and compliance using a barostat (30). The ready availability of knockin and knockout mice make the mouse model an attractive model to dissect out the pathways that contribute to fundic accommodation. Testing and validation of a method to accurately measure in vivo changes in murine fundic size and determination of the effect of cholinergic, nitrergic and serotonergic pathways on fundic size in the intact innervated murine stomach is therefore of considerable interest.
In the present study we utilized an ultrasound technique, known as ultrasonomicrometry, to determine changes in distance between piezoelectric crystals attached to the serosal surface of the murine fundus. The methodology was used to determine the effect of modulation of the major pathways that control fundic size in larger intact animals.
Ultrasonomicrometry has previously been successfully used to determine changes in volume in the murine heart (22, 23) and the rat gastric body (1, 2). As the piezoelectric crystals are small, several can be placed on the serosa of the murine stomach allowing the measurement of ultrasound waves as they pass through the thin wall of the murine fundus without needing to traverse the air-filled lumen. This avoids the current major limitation of conventional transabdominal ultrasonography. Our results show that ultrasonomicrometry can be successfully used to measure fundic size in vivo and that the measured changes accurately reflect changes in volume. These data suggest that the technique may be of use in small animal in vivo research on fundic and gastric volumes.
Methods
Animals
All experiments were approved by the Mayo Institutional Animal Care and Use Committee. Adult (6-8 weeks) male 129 SvEv mice (Taconic, Gemantown, NY), body weight 20 to 25g were used in all experiments. Their diet was changed to Ensure Plus (Abbott laboratories, Columbus, OH) with free access to water 48 hours before each experiment to make sure the stomach was empty of solid food at the time of the experiment. Mice were anesthetized with ketamine 100 mg/kg im and xylazine 10 mg/kg im. Every 40 min, half of the initial dosage was readministered. Animals were placed on a custom built heating pad set at 38° C to control body temperature and an abdominal mid-line incision was made. A silicon catheter (OD: 0.9 mm, ID 0.6 mm) was inserted through a small incision (≈1-2 mm) made in the proximal jejunum about 3-5 cm distal to the pylorus and the residual stomach content was flushed out with saline. After emptying the stomach, the tip of the catheter was placed in an area between the fundus and corpus. The catheter was ligated in place with a silk suture that also closed the jejunal incision. The other end of the catheter was connected to a saline column. The height of the column was changed by moving the column up or down to adjust the intragastric pressure value. Pressure signals were recorded using a MicroTip pressure catheter transducer (SPR-524 connected to a TCB-600 pressure control unit; Millar Instruments, Houston, TX) and stored digitally on a personal computer.
Ultrasonomicrometry and intragastric pressure measurements
A digital ultrasound-based measurement System (TRX series 8, Sonometrics, London, Ontario) was used for this study. For each experiment, 2 to 4 piezoelectric crystals (external diameter 1 mm) were glued (VetBond, 3M, St Paul, MN) to the serosal side of fundus, at least 3 mm apart (Fig 1) and in specific experiments, to the antrum. Measurements were taken every 15 ns. With a sound speed of 1.54 mm/μs through tissue, the resolution was 24 μm. An oscilloscope was used to adjust the sensitivity of the receivers to capture the first received signal. As each piezoelectric crystal acted as both an emitter and receiver, data were collected from each possible permutation (1:2, 1:3, 1:4, 2:3, 2:4, etc) between the 4 piezoelectric crystals. Data were recorded on a personal computer in real time, using the provided software (Sonoview, Sonometrics, London, Ontario). Post-acquisition processing used the same software.
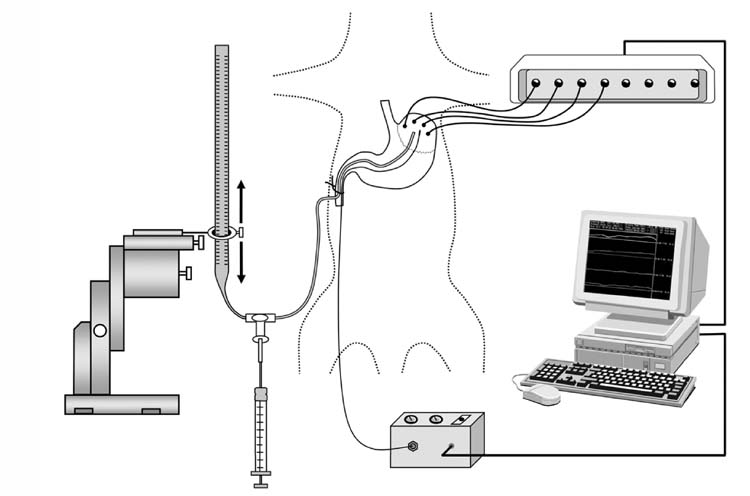
Figure 1.
Diagram of the ultrasonomicrometry system and the experimental model. Crystals (1.0 mm diameter, resolution 24 μM) were glued to the surface of stomach and distances between the crystals collected, displayed and analyzed on a windows based computer. Prior to the measurements a flexible catheter was placed through the jejunum into stomach and placed between the fundus and body to adjust intragastric pressure manually. A pressure control unit is also included in the sketch, from which a MicroTip pressure transducer was inserted into mouse stomach along with the catheter. Pressure signals were also displayed on the computer monitor.
Intragastric pressure was recorded simultaneously with the data from the piezoelectric crystals and displayed concomitantly to determine the temporal relationship between intragastric pressure and changes in fundic size as measured by changes in the distance between the crystals. Intragastric pressure was adjusted by infusion of saline through the catheter. The pressure transducer was calibrated before each experiment using a water column (figure 1).
Experimental protocols
After stable traces from the piezoelectric crystals were established, drugs were injected via the tail vein or intramuscularly. When more than one drug was used, a minimum of 20 min separated administration of each drug.
For the ex-vivo experiments the mouse stomach (n=5) was removed and placed in normal Krebs solution (at 38°C) bubbled with 3% CO2 and 97% O2. A pair of crystals was glued to the fundus (4-7 mm apart). The distal esophagus was ligated with a silk suture and the proximal duodenum connected to a 1ml syringe. Boluses of saline (100 μl) were injected into the stomach in a stepwise fashion. The changes in distance between the crystals was recorded digitally and plotted against the injected volume. In the in vivo experiments the mice (n=5) were anesthetized, the stomach exposed and the crystals placed on the fundus in situ. A similar protocol to the one described above was then used.
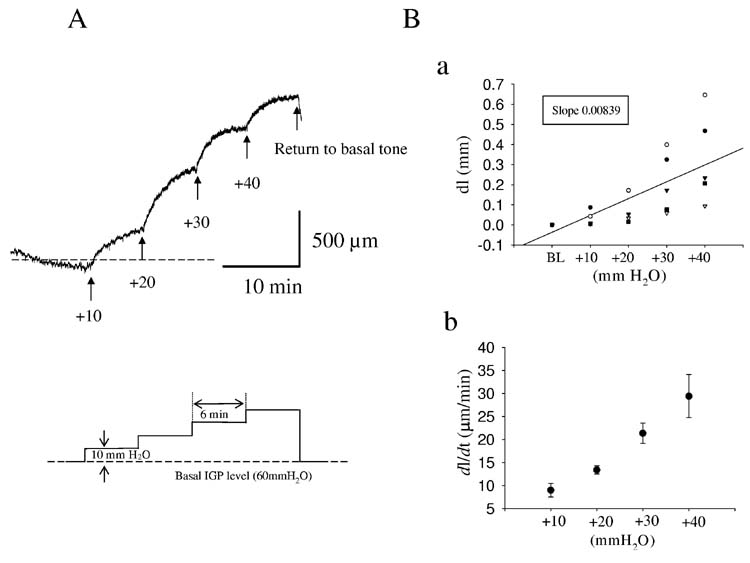
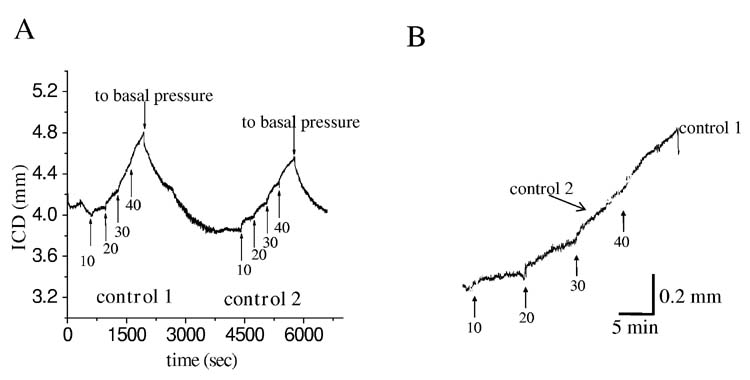
To measure compliance, intragastric pressure (IGP) was adjusted at the beginning of an experiment to 60 mmH2O (≅4.41mmHg) by infusing pre-warmed saline solution into the stomach. 60 mmH2O was chosen as the initial intragastric pressure as, in agreement with the literature (30), at this pressure both contractions and relaxations could be most easily observed. After a 10-15 min equilibration period, the IGP was increased in a stepwise fashion by 10 mmH2O (0.73 mmHg) to a maximum of 100 mm H2O (fig 2). At least 6 min separated each increase in IGP. The 6 min time period was chosen based on preliminary experiments that showed that steady state was reached in this time period. Changes in distances between crystals (dl) and IGP were continuously recorded and the inter-crystal distance (ICD) and the change in IGP (10 mmH2O) used to calculate compliance (dl/dp) and plotted (fig 2Ba).
Figure 2.
Experimental protocol used to determine compliance in the mouse fundus. After basal intragastric pressure (IGP) was set at 60 mmH2O, the IGP was increased by 10 mmH2O increments every 6 min up to 100 mmH2O. Panel A shows a typical trace and the protocol used. Panel B (upper) shows the relationship between IGP and intercrystal distance for 5 control mice. The slope of the regression line represents the compliance of the fundus. Panel B (lower) shows the relationship between the fundic distension rate (FDR) and the IGP. Numbers in panel A are in mmH20.
The gastric fundus is not only compliant but also actively relaxes to accommodate food. Disorders in fundic function may therefore not only include abnormal compliance but also impaired, delayed or slowed receptive relaxation. In order to quantify the rate of relaxation, we also measured the rate of the change in distance between the crystals, ie the fundic distension rate (FDR) in response to a given initial pressure. The FDR was calculated from dl/dt. Values of dl/dt were determined by calculating the fit of the slope of the trace (25-75%) in response to any given pressure change (fig 2Bb).
Drugs
Atropine sulphate, bethanechol chloride and Nω-Nitro-L-arginine (L-NNA) were purchased from Sigma (Sigma-Aldrich Chemical Co., St. Louis, MO). Nitroglycerin was purchased from ARL (American Regent Laboratory, CA). Buspirone hydrochloride was purchased from Tocris (Tocris Cookson Inc, Ellisville, MO) and sumatriptan succinate was purchased from GlaxoWellcome (GlaxoWellcome Inc, Research Triangle Park, NC).
All drugs were given intravenously via the tail vein, except for atropine which was given intramuscularly. The doses used were: atropine 0.2-0.4 mg/kg; bethanechol 0.15 mg/kg; sumatriptan 0.1-0.3 mg/kg; buspirone 0.1-0.3 mg/kg; nitroglycerin 0.03 mg/kg; and L-NNA 0.5-1.5 mg/kg. Doses were selected based on our previous work and on published data (5, 6, 10, 15, 17, 20, 26, 42)
Statistical analysis
All results are reported as the mean ± standard error of the mean. The number of individual experiments is indicated by the n value. Statistical significance was determined using paired student T-tests for changes in intercrystal distance in response a drug. An unpaired T-test was used for the compliance and rate of fundic distention data. A p value of less than 0.05 was considered significant.
Results
Validation studies
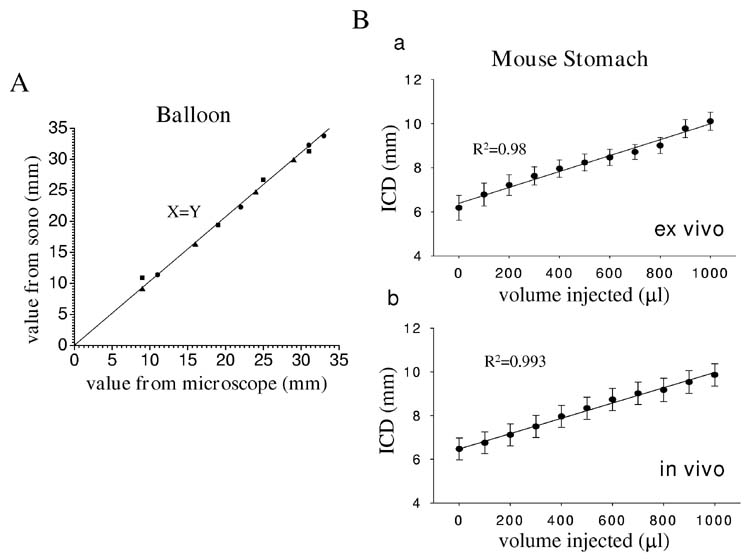
A series of validation studies were carried out to determine if the recorded values for the change in distance between the crystals correlated to the actual changes in the distance between the crystals. In the first set of experiments, the upper part of a finger of a rubber glove was cut off and attached to the tip of a 12 ml syringe. Two crystals were glued on the glove tip 10 mm apart using VetBond. The setup was placed under a dissecting microscope equipped with a micrometer. The syringe was used to inject water into the glove tip and the distance between the two crystals at different injected volumes directly measured and also measured using the ultrasonomicrometry system (n=3). As can be seen (Figure 3) there was a 1:1 correlation between the two measurements suggesting that the ultrasonomicrometry measurements accurately reflected changes in distance between the two crystals.
Figure 3.
In vitro, ex vivo, and in vivo validation of the accuracy of ultrasonomicrometry. Panel A shows the correlation between distance between two crystals attached to a glove tip, measured using a micrometer, to changes in distance measured using the ultrasonomicrometry system (n=3). A 1:1 ratio was obtained suggesting that ultrasonomicrometry accurately measured distance. Panel Ba shows the correlation between distance between two crystals placed across the dome of the fundus and volume changes in an isolated stomach (n=5). Panel Bb shows a similar experiment but in vivo with the crystals placed on the stomach after laparotomy (n=4). Known volumes of saline were added to the isolated mouse stomach to induce volume changes. There was good correlation between the two measurements in both experiments. ICD: intracrystal distance.
A second validation study was carried out to determine the correlation between the changes in distance between two crystals placed on the curved surface of the mouse fundus and the volume of the stomach. This set of experiments was carried out both ex vivo on the excised stomach and in vivo as outlined in the methods. As can be seen in Figure 3B increases in gastric volume within the physiological range of the murine stomach also resulted in proportional changes in linear distance between two crystals (n=5 for each experiment).
Pharmacological Modulation
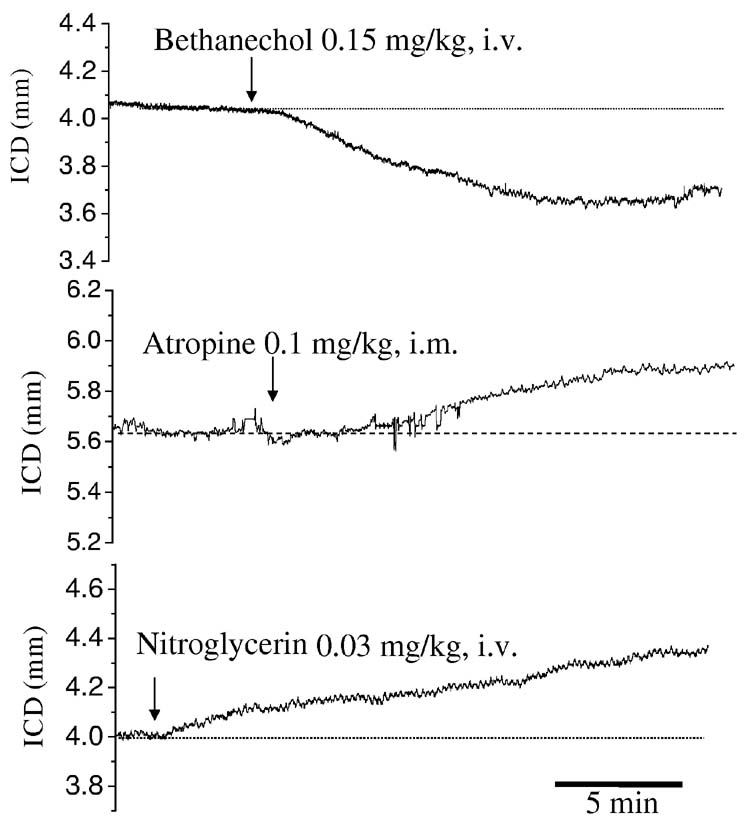
In a third set of experiments, to determine the biological responsiveness of the technique, bethanechol was used to contract the fundus and nitroglycerin, a nitric oxide donor, was used to relax the fundus (fig 4). These experiments were carried out in vivo. Bethanechol (0.15 mg/kg iv) caused a rapid decrease in the distance between the crystals, indicating fundic contraction (9.7±2.9%, fig 4, n=3, p<0.05). Nitroglycerin (0.03 mg/kg iv) caused an increase in the distance between the crystals indicating relaxation (4.6±1.8%, fig 4, n=5, P<0.05). Atropine (0.2 mg/kg im) also caused an increase in the distance between the crystals (5.5±0.9%, fig 4, n=8, p<0.05) suggesting that there was endogenous cholinergic input to baseline fundic tone.
Figure 4.
Representative traces of changes in intercrystal distance during administration of bethanechol (A), atropine (B) and nitroglycerin (C). Initial IGP was set at 60 mmH2O. An upward deflection reflects an increase in intercrystal distance (increased fundic size) and a downward deflection a decrease in intercrystal distance. Bethanechol decreased the intercrystal distance suggesting it decreased fundic size while nitroglycerin administration increased the intercrystal distance suggesting it increased fundic size. Atropine also increased the intercrystal distance suggesting a baseline cholinergic regulation of fundic tone.
It is possible that a change in fundic volume may reflect a passive response to a contraction or relaxation in other regions of the stomach. A strong antral contraction may displace enough gastric content proximally to distend the fundus. To investigate this possibility, an additional two crystals were placed on the antral surface. Changes in fundic inter-crystal distance were not accompanied by inverse changes in the antrum suggesting that the results represented a direct effect of the drugs on the fundus (data not shown).
Reproducibility
We next determined the reproducibility of our measurements. In this set of experiments, intragastric pressure was increased in a stepwise fashion from 60 to 100 mmH2O as previously described and distance between the fundic crystals measured (fig 5). The intragastric pressure was then returned to baseline and the experiment repeated. As can be see in figure 5 there was close to 1:1 correlation both between the rate of change and absolute change in distances between the two sets of data.
Figure 5.
Reproducibility of compliance measurements obtained by stepwise increases in intragastric pressure (IGP). Panel A shows changes in intercrystal distance induced by stepwise changes in IGP. After 30 minutes the protocol was repeated and the slope of both experiments plotted in Panel B. The slopes were nearly identical suggesting excellent reproducibility. Numbers are in mmH20.
In vivo measurement of changes in baseline fundic volume
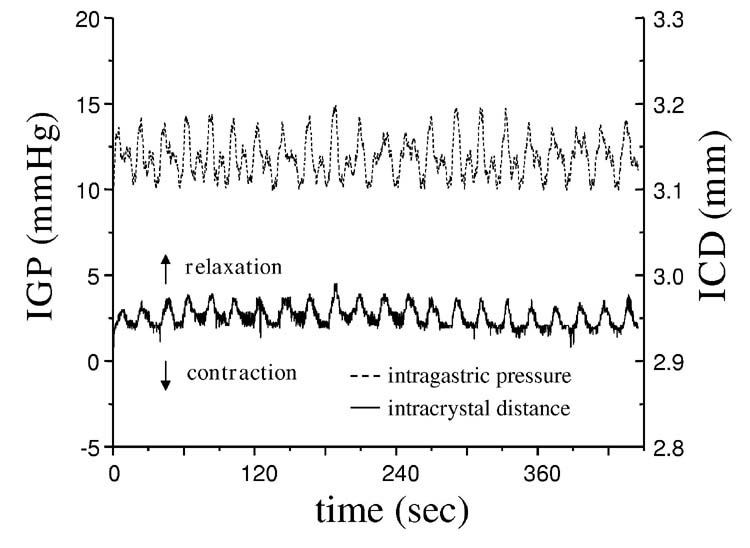
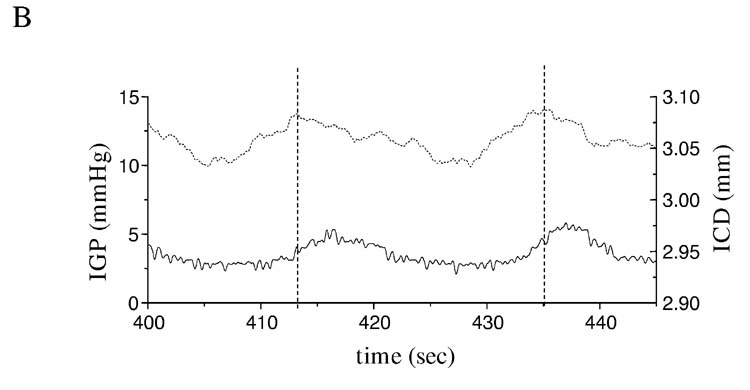
As described in the methods section, after the midline incision and placement of the crystals, the anesthetized mice were left to recover for about 30 min when the traces from all the channels reached a stable level. In about half of all animals assessed, regular oscillations in intercrystal distance, reflecting a change in fundic size, accompanied by changes in IGP (fig 6A) were recorded. These oscillations initially suggested spontaneous fundic contractions. Changes in intercrystal distance were about 25 to 33% of those recorded from the antrum (data not shown). However, peaks in IGP preceded each increase in the distance between the crystals (fig 6B). The peaks in apparent fundic contraction (smallest distance between the crystals) coincided with the lowest IGP recordings suggesting that the observed ‘contractions’ superimposed on the slower changes in fundic size reflected contractile changes in the distal stomach and not spontaneous fundic ‘contractions’.
Figure 6.
Oscillations in fundic intercrystal distance associated with changes in intragastric pressure (IGP). Panel A shows spontaneous rhythmic changes in intercrystal distance associated with changes in IGP. Panel B shows an expanded trace to highlight the temporal relationship between the apparent fundic contractions and IGP. Peak IGP (dashed line) occurred before peak changes in intercrystal distance and was lowest when intercrystal distance was lowest suggesting that the observed rhythmic activity observed in the fundus, superimposed on slower changes, reflected a passive response to contractions in the distal stomach.
In vivo measurement of compliance (dl/dp)
Fundic compliance was first determined in controls. Fundic compliance was 9.1±0.69 μm/mmH2O (n=26 preparations). As drugs were administered i.v. in 15-30 μl of saline, we tested the effect of 25 μl NaCl 0.9% (i.v) on fundic compliance. No effect was noted (table 1). We tested the effects of atropine, bethanecol and L-NNA on fundic compliance to determine the effect of cholinergic and nitrergic input on compliance. Atropine (0.4 mg/kg im) immediately increased the size of the fundus, but did not alter compliance (table 1). Bethanechol (0.15 mg/kg i.v.) and L-NNA (1.5 mg/kg i.v.) significantly reduced fundic compliance (table 1).
Table 1.
Effect of drugs on fundic compliance (dl/dP)
| Saline (25 μl, i.v.) | Sumatriptan (0.1 mg/kg. i.v.) | Buspirone (0.1 mg/kg, i.v.) | Atropine (0.4 mg/kg, i.m.) | Bethanechol (0.15 mg/kg, i.v.) | L-NNA (1.5mg/kg, i.v.) | |
|---|---|---|---|---|---|---|
| Control | 9.2±2.6(3) | 7.8±1.6(9) | 9.9±2.8(4) | 9.1±2.5(4) | 8.7±1.8(3) | 9.3±1.1(3) |
| Expt | 8.9±1.8(3) | 10.4±1.8(9)* | 8.4±8.4(4) | 7.7±1.4(4) | 3.4±0.8(3)* | 3.7±1.3(3)* |
Values are μm/mmH2O. Numbers shown in parenthesis indicate number of animals
=P<0.05 compared to control
In vivo measurement of fundic distension rate (dl/dt)
The rate of fundic distension was calculated from the slope of each trace at each intragastric pressure. Bethanechol and atropine were used to assess the influence of cholinergic pathways and L-NNA to assess the influence of nitrergic pathways on the fundus. The results before and after administration of each drug are summarized in Table 2. As previously shown, atropine immediately increased intercrystal distance but did not alter the rate of fundic distension. Bethanecol and L-NNA reduced the rate of fundic distension.
Table 2.
Effects of drugs on fundic distension rate (dl/dt)
| IGP (mmH2O) | ||||
|---|---|---|---|---|
| 10 | 20 | 30 | 40 | |
| control | 12.4±4.9 (9) | 11.6±3.9 (9) | 26.8±8.9 (9) | 37.5±10.5 (9) |
| Sumatriptan (0.1 mg/kg, iv) | 7.1±1.2 (9) | 24.6±5.8 (9)** | 49.3±12.8 (9)* | 59.2±12.9 (9)** |
| control | 9.9±5.9 (4) | 15.8±7.6 (4) | 21.3±9.1 (4) | 34.2±13.7 (4) |
| Buspirone | 12.6±8.0 (4) | 24.1±20 (4) | 30.8±21.8 (4) | 41.1±17.3 (4) |
| (0.1 mg/kg, iv) | ||||
| control | 5.3±4.4 (4) | 13.0±11.4 (4) | 21.1±15.7 (4) | 29.8±19.1 (4) |
| Atropine | 4.9±1.3 (4) | 11.5±0.7 (4) | 16.7±7.7 (4) | 30.8±18.2 (4) |
| (0.4 mg/kg, im) | ||||
| control | 8.4±3.0 (3) | 13.2±2.6 (3) | 16.2±1.0 (3) | 16.1±1.8 (3) |
| Bethanechol | −21.6±17.8 (3) | 0.7±1.5 (3)* | 3.0±0.6 (3)* | 7.2±1.8 (3)** |
| (0.15 mg/kg, iv) | ||||
| control | 15.5±3.1 (5) | 24.5±3.4 (5) | 38.1±4.8 (5) | 43.8±5.1 (5) |
| L-NNA | −2.4±1.4 (3)* | −1.2±1.5 (3)* | 10.1±2.3 (3)* | 7.4±3.5 (3)** |
| 1.5 mg/kg, iv) | ||||
Intragastric pressure (IGP) is the pressure above baseline (60mmH2O)
Values shown as μm/min. Numbers in parenthesis indicate the number of animals
=P<0.05
=P<0.001
Nitrergic Relaxation of the Fundus
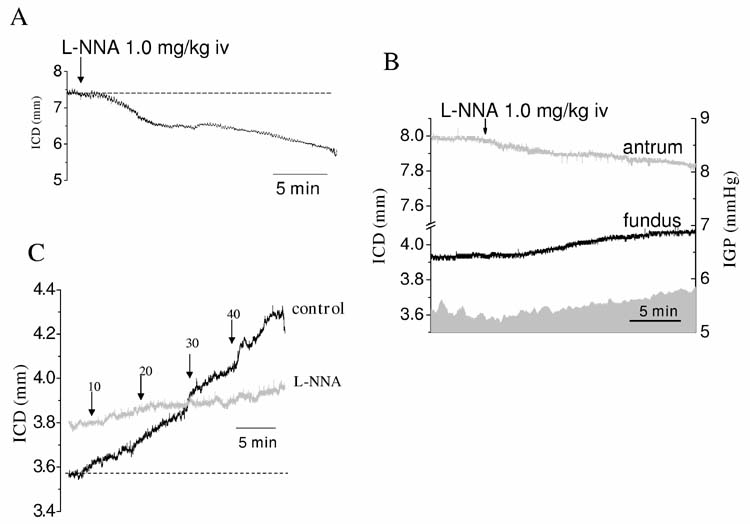
To determine the role of the nitrergic pathway in fundic relaxation in the intact mouse, we examined the effect of L-NNA on the fundus using somomicrometry in vivo. A decrease in intercrystal distance suggesting contraction of the fundus was only seen in mice in which there was no initial adjustment of the IGP to 60 mmH2O (fig 7A). In these experiments no saline was infused into the stomach and IGP left at baseline. Surprisingly, when the initial IGP was set as 60 mmH2O, administration of L-NNA (1.5 mg/kg iv), increased intercrystal distance suggesting relaxation of the fundus (2.8±0.4%, n=3, P<0.05). When distal gastric size was monitored with crystals placed on the antrum, an antral contraction was always seen with L-NNA and when intragastric pressure was measured, intragastric pressure increased on administration of L-NNA (fig 7B). It is therefore likely that the paradoxical increase in fundic size on delivery of L-NNA was due to a passive distension induced by movement of fluid from the distal stomach to the proximal stomach and that the direct effect of L-NNA on the fundus was a decrease in fundic size.
Figure 7.
Effect of the nitric oxide synthase inhibitor L-NNA on fundic tone and wall compliance. L-NNA (1.0 mg/kg iv) decreased the ICD of crystals placed on the fundus of an empty stomach with no perturbation of the initial intragastric pressure (IGP) indicating a contractile effect on fundus (A). In contrast, when the stomach IGP was set at 60 mmH2O, L-NNA increased the fundic ICD (B, black trace). This increase in ICD was preceded by a decease in the ICD of crystals placed on the antrum (B, grey trace) and by an increase in IGP (B, grey shaded area). L-NNA also decreased the compliance of gastric fundus suggesting a stiffer fundic wall (C).
As reported above, the intercrystal distance in the fundus was initially greater in the presence of L-NNA (1.5 mg/kg, i.v.), but in contrast to the pre-drug experiment, did not substantially change as IGP was increased suggesting a stiffer, non relaxing fundus in the presence of L-NNA (fig 7C).
Effect of buspirone and sumatriptan on fundic size and compliance
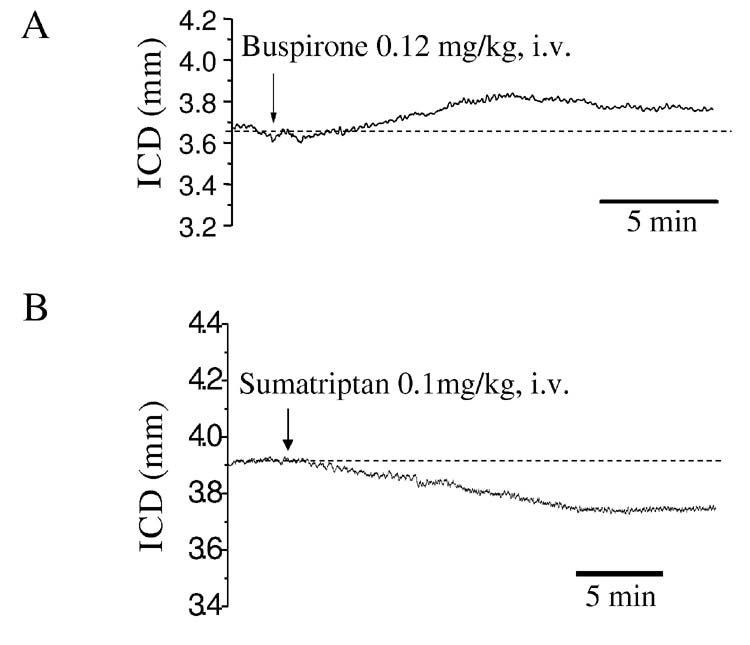
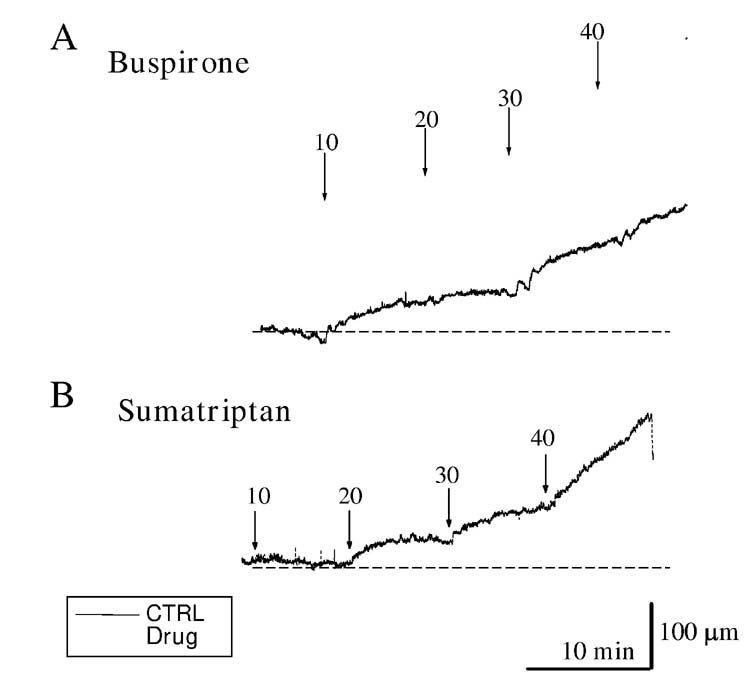
Buspirone (0.2 mg/kg, i.v.) increased the distance between crystals (3.5%±0.6%, fig 8, n=10, P<0.04), suggesting increased size of the fundus. In contrast, sumatriptan (0.1 mg/kg, i.v.) decreased the distance between crystals (2.6%±0.8% n=10, p<0.05) suggesting a decrease in fundic size (fig 8). The discrepancy between our results with sumatriptan and results using the barostat in human and larger animals which show fundic relaxation (8, 11) prompted us to further investigate the actions of both drugs on compliance and rate of fundic relaxation. Sumatriptan increased both the compliance (dl/dp, 7.8±1.6 vs 10.4±1.9 μm/mmH2O, n=9, P<0.05, Table 1) and the rate of fundic distension to given pressures (dl/dt, Table 2). The results from a typical experiment are shown in figure 9. Although sumatriptan decreased intercrystal distance, suggesting it contracted the fundus, both compliance (dl/dp) and the rate of fundic distension to a given pressure (dl/dt) were increased. In contrast, buspirone increased the distance between the crystals suggesting an increase in fundic size but did not subsequently change compliance (dl/dp, 9.9±2.8 vs 8.4±8.4 μm/mmH2O, n=4 P>0.05) nor was there any significant change in the rate of fundic distension to a given pressure (dl/dp and dl/dt, see Table 1 and Table 2 for details).
Figure 8.
Representative traces of the effect of the 5-HT1A receptor agonist buspirone and 5-HT1B/D agonist sumatriptan on murine fundus. Initial IGP was set at 60 mmH2O. Buspirone (0.12 mg/kg, i.v.) caused an increase in intercrystal distance suggesting an increase in fundic size while sumatriptan (0.1mg/kg, i.v.) decreased intercrystal distance suggesting a decrease in fundic size.
Figure 9.
Effect of the 5-HT1A receptor agonist buspirone and 5-HT1B/D agonist sumatriptan on fundic compliance. Buspirone (Panel A, pre-drug: black line, post-drug: grey line, 0.15 mg/kg, i.v.) initially increased intercrystal distance suggesting it increased fundic size but did not subsequently alter compliance. Sumatriptan (0.1 mg/kg, i.v.) initially decreased intercrystal distance suggesting it increased fundic size but, in contrast with buspirone, subsequently increased fundic compliance (Panel B, pre drug: black line, post drug: grey line).
Discussion
The main aims of the present study were to determine if ultrasonomicrometry can be used to measure changes in mouse fundic size and to determine the effect of modulation of cholinergic, nitrergic and serotonergic pathways on fundic size and compliance in the intact innervated murine stomach. Our results show that ultrasonomicrometry accurately measures changes in fundic size and is able to capture changes in size induced by pharmacological interventions. Our results also show that there is a baseline cholinergic tone in the murine fundus, and that nitrergic and serotonergic pathways affect fundic size and compliance.
Ultrasonomicrometry has advantages compared to current methodologies used to determine changes in fundic size. The technique can be applied to small animals as the spatial resolution is very good and the individual piezoelectric crystals are small. The spatial resolution in ultrasonomicrometry is determined by the speed of the sound energy and the time intervals at which the transit of the signal is measured. The transit speed of sound in most biological material is 1.54 mm/μsec. The equipment used recorded data every 15 nsec giving a spatial resolution of about 24 μm. This resolution allows measurement of small changes in the size of the fundus, not usually apparent with other methods. The crystals used in the present study were 1 mm in diameter allowing several to be placed on the surface of the murine fundus and body of the stomach. Each crystal serves as both a receiver and a transmitter enabling distances to be calculated from any one crystal to any other crystal. The crystals measure the sound signal as it passes through the wall of the organ studied thereby avoiding the problem faced by conventional ultrasonography, which relies on sound energy transmission through the whole organ. Therefore air within the stomach, particularly the fundus which often contains air, does not interfere with the ability to record the signal or to determine fundic size. A significant advantage over current methodology is that the technique can be used in small animals with intact extrinsic innervation. This is of particular importance when studying physiological processes such as fundic accommodation that requires intact vago-vagal reflexes (4, 35, 39). These reflexes are lost in muscle strip experiments or in experiments on the isolated stomach.
Fundic compliance (dl/dp) measures the deformability of the fundus by measuring the volume change resulting from a pressure change. Compliance is widely used to evaluate the distensibility of hollow organs such as the gastrointestinal tract, lungs, heart, and bladder. In the present study, in addition to measuring compliance, we also report on another coefficient, the fundic distension rate (FDR, dl/dt) which was used to provide a measure of the kinetics of distensibility. The value of the FDR is inversely proportionate to the dynamic changes in resistance to distensibility of the fundic wall. This resistance has a passive component from structures that make up the fundic wall and an active component as a result of interactions between enteric nerves, interstitial cells of Cajal and smooth muscle cells. The FDR coefficient provides information in addition to the fundic compliance coefficient as it is an expression of compliance without requiring a static measure of maximum distension. FDR gives a measure of the rate of distension in response to a given pressure. As was seen in Table 2, FDR varied at different pressure points with a three-fold increase in FDR at 40 mmH2O when compared to 10 mmH2O likely reflecting active accommodation, thereby reducing resistance to distension.
As we developed the techniques required to measure changes in fundic size we encountered limitations to the methodology. Our studies were carried out in the acute setting with anaesthetized mice. In separate experiments (data not shown), we used mouse antral muscle strips to determine the effect of various anesthetics on contractile activity. We tested all anesthetic drugs currently approved by our institution for use in mice, including diazepam, ketamine, pentobarbital, thiopental and thamylal at the recommended doses. All anesthetic drugs tested affected contractile activity, decreasing contractile amplitude. The combination of ketamine and xylazine had the least effect on spontaneous activity and was therefore used in this study.
Another limitation of the ultrasonomicrometry technique is that it does not directly measure IGP. This limitation was highlighted in our experiments with L-NNA as a nitric oxide synthase inhibitor. L-NNA would be expected to reduce nitric oxide production and therefore cause the fundus to contract. In contrast an apparent relaxation was seen. Use of an IGP monitoring device and placement of additional crystals on the distal body of the stomach showed that L-NNA caused contraction of the gastric body, resulting in displacement of fluid from the stiffer distal stomach to the more compliant proximal stomach thereby distending the fundus. These results suggest that it is important to monitor IGP simultaneously when using ultrasonomicrometry in all experiments. Morever, while the technique has been successfully used to isolate longitudinal and circular muscle contraction or relaxation by placing crystals along the axis of contraction (2), this is harder to accomplish in the fundus. This is due to the spherical nature of the fundus making the axis of contraction different in different parts of the fundus. We therefore did not attempt to separate out the contribution of each muscle layer to the changes observed.
The experiments directed towards determining the effect of modulation of cholinergic, nitrergic and serotonergic pathways on murine fundic size and compliance in the intact innervated stomach revealed different contributions of each pathway to regulation of fundic tone. As previously shown (38), there appears to be a baseline cholinergic input maintaining fundic tone as atropine resulted in an increase in intercrystal distance suggesting relaxation. The data obtained using L-NNA to inhibit nitric oxide production while monitoring IGP and antral size are also in agreement with those obtained in other intact animals including humans (32, 40). Furthermore L-NNA decreased fundic compliance and markedly altered the rate of fundic relaxation to a given pressure suggesting that there also was a baseline nitrergic input to fundic smooth muscle and that in the absence of nitric oxide the fundus is stiffer and non relaxing. A serotonergic modulation of fundic tone has been previously reported (27, 29, 41). In contrast to data obtained from humans (8) and dogs (12), sumatriptan did not relax the murine fundus. Instead a decrease in intercrystal distance was seen suggesting a contraction. Sumatriptan did subsequently increase fundic compliance. These data suggest that there are species differences in the serotonergic modulation of fundic size and, while different from the human and canine data, are similar to those seen in the cat where sumatriptan also contracts the cat fundus (Jan Tack – personal communication).
In summary, the validation experiments carried out in this study show that ultrasonomicrometry accurately measures distance in the mouse fundus, can be used in the intact animal, has an excellent resolution and can measure the biological responses to drugs when IGP is also monitored. The data obtained with sumatriptan and buspirone also suggest that ultrasonomicrometry can also be used to explore mechanisms of action of drugs. As highlighted by the results obtained with sumatriptan experiments, important species differences may be present in the response of the fundus to a given drug.
Acknowledgments
This work was supported by NIH grants DK 52766, DK 57061 and by a grant from Johnson & Johnson Pharmaceutical Research and Development.
References
- 1.Adelson DW, Million M. Tracking the moveable feast: sonomicrometry and gastrointestinal motility. News Physiol Sci. 2004;19:27–32. doi: 10.1152/nips.01439.2003. [DOI] [PubMed] [Google Scholar]
- 2.Adelson DW, Million M, Kanamoto K, Palanca T, Tache Y. Coordinated gastric and sphincter motility evoked by intravenous CCK-8 as monitored by ultrasonomicrometry in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G321–332. doi: 10.1152/ajpgi.00057.2003. [DOI] [PubMed] [Google Scholar]
- 3.Azpiroz F, Malagelada JR. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology. 1987;92:934–943. doi: 10.1016/0016-5085(87)90967-x. [DOI] [PubMed] [Google Scholar]
- 4.Azpiroz F, Malagelada JR. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am J Physiol. 1986;251:G727–735. doi: 10.1152/ajpgi.1986.251.6.G727. [DOI] [PubMed] [Google Scholar]
- 5.Barocelli E, Ballabeni V, Bertoni S, De Amici M, Impicciatore M. Evidence for specific analgesic activity of a muscarinic agonist selected among a new series of acetylenic derivatives. Life Sci. 2001;68:1775–1785. doi: 10.1016/s0024-3205(01)00973-0. [DOI] [PubMed] [Google Scholar]
- 6.Barocelli E, Chiavarini M, Ballabeni V, Barlocco D, Vianello P, Dal Piaz V, Impicciatore M. Study of the antisecretory and antiulcer mechanisms of a new indenopirydazinone derivative in rats. Pharmacol Res. 1997;35:487–492. doi: 10.1006/phrs.1997.0168. [DOI] [PubMed] [Google Scholar]
- 7.Berstad A. Today's therapy of functional gastrointestinal disorders--does it help? Eur J Surg Suppl. 1998:92–97. doi: 10.1080/11024159850191328. [DOI] [PubMed] [Google Scholar]
- 8.Boeckxstaens GE, Hirsch DP, Kuiken SD, Heisterkamp SH, Tytgat GN. The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol. 2002;97:40–48. doi: 10.1111/j.1572-0241.2002.05421.x. [DOI] [PubMed] [Google Scholar]
- 9.Cannon WB, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911;29:267–273. [Google Scholar]
- 10.Cao BJ, Rodgers RJ. Comparative behavioural profiles of buspirone and its metabolite 1-(2-pyrimidinyl)-piperazine (1-PP) in the murine elevated plus-maze. Neuropharmacology. 1997;36:1089–1097. doi: 10.1016/s0028-3908(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 11.Coulie B, Tack J, Sifrim D, Andrioli A, Janssens J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am J Physiol. 1999;276:G373–377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- 12.De Ponti F, Crema F, Moro E, Nardelli G, Frigo G, Crema A. Role of 5-HT1B/D receptors in canine gastric accommodation: effect of sumatriptan and 5-HT1B/D receptor antagonists. Am J Physiol Gastrointest Liver Physiol. 2003;285:G96–104. doi: 10.1152/ajpgi.00280.2002. [DOI] [PubMed] [Google Scholar]
- 13.De Schepper HU, Cremonini F, Chitkara D, Camilleri M. Assessment of gastric accommodation: overview and evaluation of current methods. Neurogastroenterol Motil. 2004;16:275–285. doi: 10.1111/j.1365-2982.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 14.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- 15.DiMagno MJ, Hao Y, Tsunoda Y, Williams JA, Owyang C. Secretagogue-stimulated pancreatic secretion is differentially regulated by constitutive NOS isoforms in mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G428–436. doi: 10.1152/ajpgi.00368.2003. [DOI] [PubMed] [Google Scholar]
- 16.Friedman G. Treatment of the irritable bowel syndrome. Gastroenterol Clin North Am. 1991;20:325–333. [PubMed] [Google Scholar]
- 17.Furfine ES, Harmon MF, Paith JE, Garvey EP. Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry. 1993;32:8512–8517. doi: 10.1021/bi00084a017. [DOI] [PubMed] [Google Scholar]
- 18.Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- 19.Gershon MD. The enteric nervous system: a second brain. Hosp Pract (Off Ed) 1999;34:31, 35, 41–32. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- 20.Ghelardini C, Galeotti N, Grazioli I, Uslenghi C. Indomethacin, alone and combined with prochlorperazine and caffeine, but not sumatriptan, abolishes peripheral and central sensitization in in vivo models of migraine. J Pain. 2004;5:413–419. doi: 10.1016/j.jpain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Grey E. Observations on the postural activity of the stomach. Am J Physiol. 1918;45:272–285. [Google Scholar]
- 22.Guth BD, Schulz R, Heusch G. Time course and mechanisms of contractile dysfunction during acute myocardial ischemia. Circulation. 1993;87:IV35–42. [PubMed] [Google Scholar]
- 23.Hoit BD. New approaches to phenotypic analysis in adult mice. J Mol Cell Cardiol. 2001;33:27–35. doi: 10.1006/jmcc.2000.1294. [DOI] [PubMed] [Google Scholar]
- 24.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 25.Jahnberg T, Abrahamsson H, Jansson G, Martinson J. Gastric relaxatory response to feeding before and after vagotomy. Scand J Gastroenterol. 1977;12:225–228. doi: 10.1203/00006450-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Kawabata A, Nishikawa H, Kuroda R, Kawai K, Hollenberg MD. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br J Pharmacol. 2000;129:1808–1814. doi: 10.1038/sj.bjp.0703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima S, Ishizaki R, Shimo Y. Investigation into the 5-hydroxytryptamine-induced relaxation of the circular smooth muscle of guinea-pig stomach fundus. Eur J Pharmacol. 1992;224:45–49. doi: 10.1016/0014-2999(92)94816-e. [DOI] [PubMed] [Google Scholar]
- 28.Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest. 1996;98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moen H, Ertresvaag K, Gerner T. Motor responses to serotonin in isolated guinea pig fundus and antrum. Scand J Gastroenterol. 1983;18:145–149. doi: 10.3109/00365528309181574. [DOI] [PubMed] [Google Scholar]
- 30.Monroe MJ, Hornby PJ, Partosoedarso ER. Central vagal stimulation evokes gastric volume changes in mice: a novel technique using a miniaturized barostat. Neurogastroenterol Motil. 2004;16:5–11. doi: 10.1046/j.1365-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 31.Selemidis S, Cocks TM. Nitrergic relaxation of the mouse gastric fundus is mediated by cyclic GMP-dependent and ryanodine-sensitive mechanisms. Br J Pharmacol. 2000;129:1315–1322. doi: 10.1038/sj.bjp.0703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark ME, Szurszewski JH. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992;103:1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- 33.Tack J, Demedts I, Meulemans A, Schuurkes J, Janssens J. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut. 2002;51:219–224. doi: 10.1136/gut.51.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504( Pt 2):479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thumshirn M, Camilleri M, Hanson RB, Williams DE, Schei AJ, Kammer PP. Gastric mechanosensory and lower esophageal sphincter function in rumination syndrome. Am J Physiol. 1998;275:G314–321. doi: 10.1152/ajpgi.1998.275.2.G314. [DOI] [PubMed] [Google Scholar]
- 37.Vu MK, Straathof JW, v d Schaar PJ, Arndt JW, Ringers J, Lamers CB, Masclee AA. Motor and sensory function of the proximal stomach in reflux disease and after laparoscopic Nissen fundoplication. Am J Gastroenterol. 1999;94:1481–1489. doi: 10.1111/j.1572-0241.1999.1130_f.x. [DOI] [PubMed] [Google Scholar]
- 38.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilbur BG, Kelly KA. Effect of proximal gastric, complete gastric, and truncal vagotomy on canine gastric electric activity, motility, and emptying. Ann Surg. 1973;178:295–303. doi: 10.1097/00000658-197309000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci U S A. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue L, Locke GR, Schuurkes JAJ, Meuleman A, Coulie BJ, Szurszewski JH, Farrugia G. Serotonergic modulation of murine fundic tone (Abstract) Gastroenterol. 2003;124:A580. doi: 10.1152/ajpgi.00224.2006. [DOI] [PubMed] [Google Scholar]
- 42.Yuan R, Sumi M, Benet LZ. Investigation of aortic CYP3A bioactivation of nitroglycerin in vivo. J Pharmacol Exp Ther. 1997;281:1499–1505. [PubMed] [Google Scholar]