Abstract
In this review article, the roles of imaging with CT and MRI in the detection and staging of pancreatic carcinoma will be discussed. The frequently employed techniques using these modalities, the common imaging appearances of this tumor, and the limitations of imaging will be addressed.
Keywords: Computed tomography, magnetic resonance imaging, pancreas neoplasms, pancreas CT, pancreas MRI
Introduction
Pancreatic adenocarcinoma is the most common non-endocrine malignancy of the pancreas and is the 4th leading cause of death is the United States. Most tumors arise in the head of the pancreas, and account for between 60 and 70% of cases [1–4]. Despite the recent advances in imaging and treatment, pancreatic adenocarcinoma continues to be a lethal disease. While newer diagnostic techniques have improved the accuracy for detecting these tumors, no significant inroads have been made in finding ‘early’ cancers. Most tumors are diagnosed late and approximately 85% of tumors are unresectable at the time of diagnosis. There are many reasons for this fact, but pancreatic carcinoma is unique in several respects:
(1) Symptoms manifest late
(2) Early extrapancreatic spread of tumor
(3) Rapid downhill course from diagnosis to death.
Role of imaging
The role of imaging in patients with suspected pancreatic carcinoma is:
- (1) Confirm and stage tumor
- (a) Determine if tumor is resectable or not
- (b) Exclude pancreatic carcinoma in patients with symptoms suggestive of disease.
TNM staging
| T0 | No tumor |
| T1 | Tumor confined to pancreas |
| T1a | Tumor <2 cm |
| T1b | Tumor >2 cm |
| T2 | Tumor extension into duodenum, bile duct |
| or peripancreatic tissues | |
| T3 | Tumor extension into stomach, colon, or |
| adjacent great vessels | |
| N0 | No regional nodal metastases |
| N1 | Regional nodal metastases |
| M0 | No distant metastases |
| M1 | Distal metastases. |
While the criteria for unresectability vary from center to center, the presence of distant disease (metastases), local tumor extension, documented regional or distant lymph node metastases, and arterial invasion or encasement of major mesenteric arteries (celiac, hepatic, superior mesenteric artery) are generally accepted as criteria of unresectability. Venous involvement of the major mesenteric veins (superior mesenteric vein and portal vein) is not universally accepted as a criterion of unresectability as surgeons are performing en-block venous resection and venous reconstructions.
Computed tomography (CT) techniques
CT techniques in assessment of patients with suspected pancreatic carcinoma usually involve the use of thin section dynamic contrast-enhanced helical CT obtained during the rapid bolus injection of large amounts of iodinated urographic contrast.
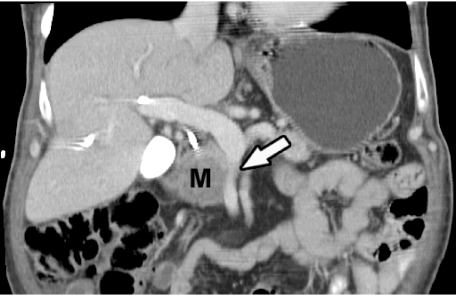
The introduction of multidetector-row scanners has facilitated the acquisition of images during multiple phases of intravenous contrast administration. Utilization of a pancreatic parenchymal phase, using a scan delay of 40 s has resulted in superior pancreatic parenchymal enhancement. In some studies, this has led to superior tumor-to-parenchymal contrast differences, facilitating superior tumor detection, when compared to portal venous or delayed phases of imaging [5–9]. The information obtained from these multiphase exams was used to generate 3D images of the arterial, venous and pancreatico-biliary anatomy [10–14]. These in select cases are useful for surgical planning (Fig. 1).
Figure 1.
Coronal multiplanar reformatted CT image shows pancreatic adenocarcinoma (M) with superior mesenteric vein encasement (arrow).
CT appearances
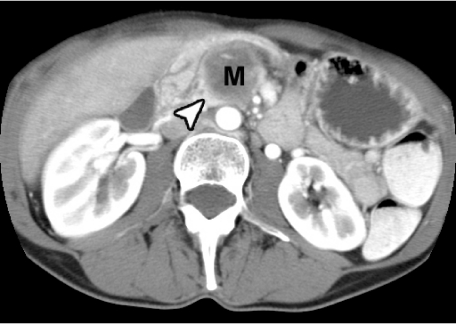
Most pancreatic adenocarcinomas are of lower attenuation than the normally enhancing pancreatic parenchyma in all phases of contrast enhancement (Fig. 2). About 10% of pancreatic adenocarcinomas can be isoattenuating on CT. Pancreatic and bile duct dilatation are also common findings, as is atrophy proximal to the tumor. Perivascular tumor extension which leads to vascular involvement and arterial or venous encasement are also hallmarks of this tumor. In pancreatitis (either acute or chronic), they are usually streaky ill-defined areas of perivascular infiltration, whereas with pancreatic carcinoma it is usually seen as a ‘cuff’ of soft tissue encasing the peripancreatic vessels.
Figure 2.
Pancreatic carcinoma (M) is low density in nature, as compared to enhancing normal pancreas (arrowhead).
CT: tumor detection and staging
While CT is excellent in detecting unresectable tumors (>90% accuracy), it frequently understages true tumor extent and even with early helical CT, the accuracy for assessing resectability was only around 70% [4]. With the use of newer multislice helical CT scanners, tumor detection rates have improved to around 90–95%. However only small improvements have been seen for determining resectability status. The most recent studies using multislice scanners have shown that positive predictive values for resectability are slightly above 80% [5, 7, 8]. Reasons for this include the continued poor sensitivity for the detection of small peritoneal and liver metastases, metastases in normal sized lymph nodes and subtle peripancreatic tumor extension.
Magnetic resonance imaging (MRI) techniques
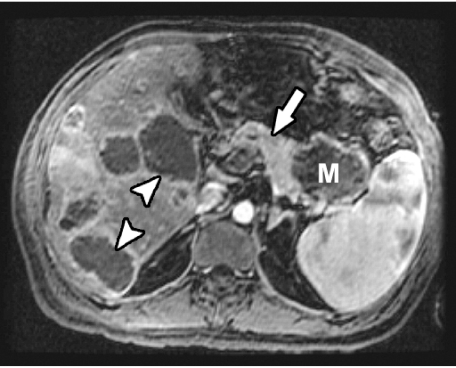
Current MR techniques using phased-array torso coils, thin slices, and dynamic gadolinium-enhanced breath-hold gradient-echo (GRE) sequences are optimal for imaging pancreatic carcinoma, and in some studies have outperformed CT especially in the detection of smaller tumors. The sequences that are most helpful for the detection of pancreatic carcinoma are the T1-weighted fat-suppressed and gadolinium-enhanced GRE sequences. On the T1-weighted fat-suppressed images, the pancreas usually due to its proteinaceous content is of high signal intensity, while the tumor is of low signal intensity. On the gadolinium-enhanced GRE images, pancreatic carcinomas enhance less than the surrounding parenchyma (Fig. 3) [15–19]. Mangafodipir trisodium-enhanced MRI has also demonstrated that when compared to contrast-enhanced CT, it was as accurate for the detection and staging of pancreatic adenocarcinoma, and slightly superior to it for the detection of small tumors and metastases [20, 21].
Figure 3.
On gadolinium-enhanced breath-held 3D spoiled GRE MR image, pancreatic carcinoma (M) in tail of pancreas does not show any significant enhancement as compared to adjacent normal pancreatic parenchyma (arrow). Note multiple hepatic metastases (arrowheads).
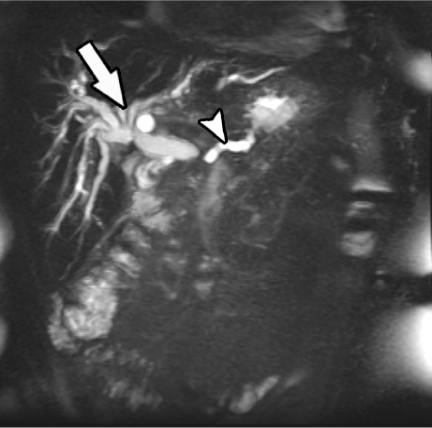
The added ability to perform magnetic resonance cholangiopancreatography (Fig. 4), as well as the ability to assess vascular involvement with use of magnetic resonance angiography, makes MRI an invaluable tool in providing a comprehensive evaluation of patients with pancreatic carcinoma, assessing local tumor extent, peripancreatic vessel involvement, pancreatico-biliary duct anatomy, as well as for distant metastases [22, 23].
Figure 4.
Single-shot fast spin-echo coronal MR image demonstrates dilated pancreatic duct (arrowhead) and dilated intrahepatic bile ducts (arrow) due to obstructing pancreatic carcinoma.
Vascular invasion
Arterial involvement
The most common vessels involved are the celiac axis, splenic artery, and superior mesenteric artery. Major arterial encasement is seen as soft tissue infiltration along the vessels resulting in a soft tissue ‘cuff’ or the appearance of a thickened vessel (Fig. 5). This finding is not specific for tumor invasion as rarely, pancreatitis may present with a similar appearance. This vascular encasement and retropancreatic invasion into the celiac plexus results in the back pain that these patients often present with.
Figure 5.
Contrast-enhanced CT shows arterial encasement due to pancreatic carcinoma seen as soft tissue infiltration around superior mesenteric artery (arrow).
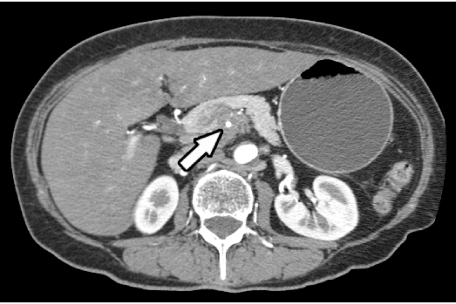
Venous involvement
The most common veins involved are splenic vein, superior mesenteric vein and the portal vein (Fig. 6). Venous invasion can be suggested if the vein is attenuated or changes its caliber. The superior mesenteric vein when attenuated by a tumor assumes a ‘tear-drop’ appearance. This sign has a high specificity for unresectability (85%) [24]. Another indirect sign of venous involvement is the presence of dilated peripancreatic collaterals. In late stages, venous occlusion and thrombosis may also be seen.
Figure 6.
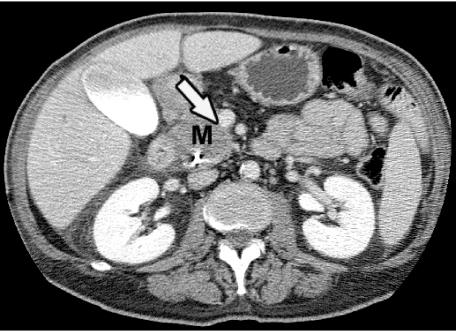
Pancreatic carcinoma (M) abutting more than 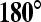 of circumference of superior mesenteric vein (arrow) is seen on contrast-enhanced CT, indicating high likelihood of encasement, which was confirmed at surgery.
of circumference of superior mesenteric vein (arrow) is seen on contrast-enhanced CT, indicating high likelihood of encasement, which was confirmed at surgery.
In the last several years, attention has been focused on the small veins of the pancreatic arcades. These veins are usually small, and lie dorsal and ventral to the head and uncinate process of the pancreas. When there is a tumor compressing the tributaries of the superior mesenteric vein or portal vein, these veins get dilated. This sign can be an indirect and early sign of an unresectable tumor [25].
Predicting resectability based on tumor contact with peripancreatic vessels
Several CT studies have been performed to determine if the degree of contact of a tumor with the adjacent major peripancreatic arteries and veins could be used to predict if a tumor could be resected or not. These studies have shown that if there is a clear fat plane or normal pancreatic parenchyma interposed between the tumor and these vessels, in almost all instances the tumor is resectable. If there was tumor contact of 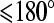 , then the likelihood of resectability was high and if the degree of tumor contact was greater than 180∘, it was most likely unresectable [26, 27].
, then the likelihood of resectability was high and if the degree of tumor contact was greater than 180∘, it was most likely unresectable [26, 27].
CT vs. MR—which modality is superior?
Recent studies have tried to compare the two techniques and have come up with divergent results. In some studies MR was superior to CT and in others the reverse was true. Due to the rapid changes in imaging technology, these results are short-lived [15, 16]. It remains to be seen if ultra thin-section dynamic contrast-enhanced multislice CT and dynamic breath-held gadolinium-enhanced 3D volume acquisitions will translate into improved tumor detection and staging.
Limitations of imaging methods
The most common reasons for understaging by imaging are the inability to detect:
(1) Metastases to normal sized lymph nodes
(2) Small peritoneal metastases
(3) Small <1 cm hepatic metastases
(4) Subtle peripancreatic tumor extension.
Most metastatic lymph nodes are <1 cm and this poses a problem for current imaging techniques as size criteria are the only method we currently have to distinguish between benign and malignant nodes. Using ultra-small iron-oxide particles for lymph node imaging with MR may offer a solution in the future. Peritoneal metastases are recognized only when in an advanced stage and small metastatic deposits still go undetected. Similarly the sensitivity for CT and MR in detecting small subcentimeter surface liver metastases is poor.
References
- 1.Kalra MK, Maher MM, Sahani DV, et al. Current status of imaging in pancreatic diseases. J Comput Assist Tomogr. 2002;26:661–75. doi: 10.1097/00004728-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Balci NC, Semelka RC. Radiologic diagnosis and staging of pancreatic adenocarcinoma. Eur J Radiol. 2001;38:105–12. doi: 10.1016/s0720-048x(01)00295-9. [DOI] [PubMed] [Google Scholar]
- 3.Tamm E, Charnsangavej C. Pancreatic cancer: current concepts in imaging for diagnosis and staging. Cancer J. 2001;7:298–311. [PubMed] [Google Scholar]
- 4.Bluemke DA, Cameron JL, Hruban RH, et al. Potentially curable pancreatic adenocarcinoma: spiral CT assessment with surgical and pathologic correlation. Radiology. 1995;197:381–5. doi: 10.1148/radiology.197.2.7480681. [DOI] [PubMed] [Google Scholar]
- 5.Diehl SJ, Lehmann KJ, Sadick M, et al. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology. 1998;206:373–8. doi: 10.1148/radiology.206.2.9457188. [DOI] [PubMed] [Google Scholar]
- 6.O’Malley ME, Boland GW, Wood BL, et al. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thin-section pancreatic-phase helical CT. Am J Roentgenol. 1999;173:1513–8. doi: 10.2214/ajr.173.6.10584794. [DOI] [PubMed] [Google Scholar]
- 7.Valls C, Sanchez AE, Fabregat J, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. Am J Roentgenol. 2002;178:821–6. doi: 10.2214/ajr.178.4.1780821. [DOI] [PubMed] [Google Scholar]
- 8.Laghi A, Iannaccone R, Catalone C, et al. Multislice spiral computed tomography in diagnosis and staging of pancreatic carcinoma: preliminary experience. Dig Liv Dis. 2002;34:732–8. doi: 10.1016/s1590-8658(02)80025-1. [DOI] [PubMed] [Google Scholar]
- 9.Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multidetector-row CT: secondary signs. Radiology. 2002;224:764–8. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- 10.Prokesch RW, Chow LC, Beaulieu CF, et al. Local staging of pancreatic carcinoma with multidetector-row CT: use of curved planar reformations—initial experience. Radiology. 2002;225:759–65. doi: 10.1148/radiol.2253010886. [DOI] [PubMed] [Google Scholar]
- 11.Catalano C, Laghi A, Fraioli F, et al. Pancreatic carcinoma: the role of high resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol. 2003;13:149–56. doi: 10.1007/s00330-002-1473-4. [DOI] [PubMed] [Google Scholar]
- 12.Horton KM, Fishman EK. Multidetector CT angiography of pancreatic carcinoma: part I: evaluation of arterial involvement. Am J Roentgenol. 2002;178:827–32. doi: 10.2214/ajr.178.4.1780827. [DOI] [PubMed] [Google Scholar]
- 13.Horton KM, Fishman EK. Multidetector CT angiography of pancreatic carcinoma: part II: evaluation of venous involvement. Am J Roentgenol. 2002;178:833–6. doi: 10.2214/ajr.178.4.1780833. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PT, Heath DG, Hofmann LV, et al. Multidetector-row computed tomography with three-dimensional volume rendering of pancreatic cancer: a complete preoperative staging toll using computed tomography angiography and volume-rendered cholangiopancreatography. J Comput Assist Tomogr. 2003;27:347–53. doi: 10.1097/00004728-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Irie H, Honda H, Kaneko K, et al. Comparison of helical CT and MR imaging in detecting and staging small pancreatic adenocarcinoma. Abdom Imaging. 1997;22:429–33. doi: 10.1007/s002619900226. [DOI] [PubMed] [Google Scholar]
- 16.Arslan A, Buanes T, Geitung JT. Pancreatic carcinoma: MR, MR angiography, and dynamic helical CT in the evaluation of vascular invasion. Eur J Radiol. 2001;38:151–9. doi: 10.1016/s0720-048x(00)00280-1. [DOI] [PubMed] [Google Scholar]
- 17.Obuz F, Dicle O, Coker A, et al. Pancreatic adenocarcinoma: detection and staging with dynamic MR imaging. Eur J Radiol. 2001;38:146–50. doi: 10.1016/s0720-048x(00)00274-6. [DOI] [PubMed] [Google Scholar]
- 18.Robinson PA. The role of MRI in pancreatic cancer. Eur Radiol. 2002;12:267–9. doi: 10.1007/s00330-001-1148-6. [DOI] [PubMed] [Google Scholar]
- 19.Fischer U, Vosshenrich R, Horstmann O, et al. Preoperative local MRI-staging of patients with a suspected pancreatic mass. Eur Radiol. 2002;12:296–303. doi: 10.1007/s00330-001-1149-5. [DOI] [PubMed] [Google Scholar]
- 20.Rieber A, Tomczak R, Nussle K, et al. MRI with mangafodipir trisodium in the detection of pancreatic tumors: comparison with helical CT. Br J Radiol. 2000;73:1165–9. doi: 10.1259/bjr.73.875.11144793. [DOI] [PubMed] [Google Scholar]
- 21.Schima W, Fugger R, Schober E, et al. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. Am J Roentgenol. 2002;179:717–24. doi: 10.2214/ajr.179.3.1790717. [DOI] [PubMed] [Google Scholar]
- 22.Lopez Hanninen E, Amthauer H, Hosten N, et al. Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology. 2002;224:34–41. doi: 10.1148/radiol.2241010798. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz LH, Lefkowitz RA, Panicek DM, et al. Breath-hold magnetic resonance cholangiopancreatography in the evaluation of malignant pancreaticobiliary obstruction. J Comput Assist Tomogr. 2003;27:307–14. doi: 10.1097/00004728-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hough TJ, Raptopoulos V, Sewert B, et al. Tear-drop superior mesenteric vein: CT sign of unresectable carcinoma of the pancreas. Am J Roentgenol. 1999;173:1509–12. doi: 10.2214/ajr.173.6.10584793. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Mori H, Kiyosue H, et al. CT assessment of the inferior peripancreatic veins: clinical significance. Am J Roentgenol. 2000;174:677–84. doi: 10.2214/ajr.174.3.1740677. [DOI] [PubMed] [Google Scholar]
- 26.Loyer EM, David CL, Dubrow RA, et al. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin section CT. Abdom Imaging. 1996;21:202–6. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 27.Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. Am J Roentgenol. 1997;168:1439–43. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]