Abstract
Despite the excellent clinical performance of fluorodeoxyglucose (FDG) as a cancer-imaging agent for positron emission tomography (PET), false positive and false negative results can be problematic in some clinical settings. Radiopharmaceutical development has recently focussed on the search for new PET tracers that could complement or replace FDG in such settings. Due to the general availability and favourable physical properties of fluorine-18, much effort has been directed to fluorinated compounds. The most promising of these are discussed.
Keywords: Positron emission tomography, fluorinated radiopharmaceuticals, cell proliferation, amino acid metabolism, choline
Positron emission tomography (PET) is being increasingly utilised for the evaluation of patients with known or suspected cancer at all phases of the diagnostic process. Globally, the vast majority of these investigations are performed using the glucose analogue, [18F]fluorodeoxyglucose (FDG). This radiotracer generally has high uptake in cancerous lesions and low uptake in benign lesions leading to both high sensitivity and specificity for the evaluation of the presence and extent of malignancy. The relatively long physical half-life and relatively low positron range of F-18 also offer practical advantages for cancer imaging.
Nevertheless, the sensitivity of FDG–PET is imperfect, not only due to the finite spatial resolution of the instrumentation but also due to poor contrast in some situations. The FDG-avidity of tumours has been shown to broadly correlate with the grade of many cancers and, a corollary of this, to provide prognostic stratification. However, some tumours with high metastatic potential can have relatively low FDG-uptake. For example, bone metastases from prostate cancer are frequently not visualised on FDG–PET. Although the relatively low background activity in most normal tissues under fasting conditions provides relatively high contrast with which to identify malignant lesions with adequate FDG-avidity, physiologic FDG uptake in normal tissues can mask cancers. The most obvious example of this is in the brain where high glucose utilisation by the normal cerebral cortex can mask brain tumours, particularly those of lower grade. Similarly, the specificity of FDG–PET is also imperfect with some benign conditions, particularly inflammatory and granulomatous lesions, having high FDG uptake [1]. These can lead to false positive results that may either prevent appropriate aggressive therapy or lead to unnecessary diagnostic interventions. These very real limitations of FDG as a cancer-imaging agent have stimulated the search for additional radiotracers to either replace or complement FDG.
There are a number of biological characteristics of malignant cells that present potential imaging targets. One of the more important of these is an increased rate of cellular proliferation. The search for proliferation markers has been active with most attenuation to thymidine analogues. [11C]Thymidine has shown promise but C-11 compounds are not very practical for routine clinical application with production yields seldom being adequate for more than a few patient studies. Consequently, researchers have looked for fluorinated analogues. The most promising of these to date has been [18F]fluoro-thymidine (FLT) [2, 3]. There is now good evidence that FLT uptake is closely correlated with cellular proliferation [4]. Since increased proliferation is not a feature of most inflammatory conditions that can cause false positive results on FDG–PET, initial studies have focussed on clinical situations where the prevalence of false positive results is clinically important. One such example has been the evaluation of solitary pulmonary nodules (SPN). Although the overall accuracy of FDG–PET for detecting malignancy of SPNs is in the order of 90%, granulomatous diseases such as tuberculosis represent an important component of the incorrect diagnostic categorisations. Preliminary data suggest that FLT may have a higher specificity than FDG in this clinical setting. The high uptake of FLT in the bone marrow (BM) related to active cellular proliferation and in the human liver potentially also limits the relative sensitivity of FLT–PET compared to FDG–PET for detection of primary or metastatic lesions in these sites. FLT is however an exciting tracer for therapeutic monitoring trials (Fig. 1).
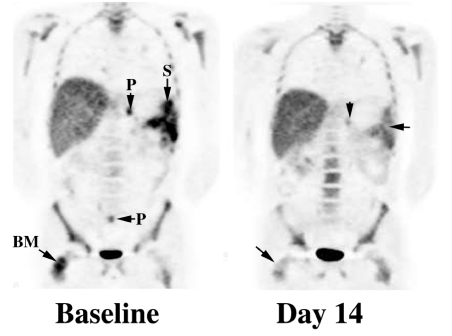
Figure 1.
Baseline FLT imaging in this patient with metastatic malignant melanoma demonstrated splenic (S), peritoneal (P) and BM metastases. After 14 days of treatment with a novel antiangiogenesis agent the upper abdominal peritoneal deposit (vertical arrow) had substantially decreased activity while the lower abdominal focus could no longer be visualised. Uptake at sites of baseline abnormality in the spleen (horizontal arrow) and right femoral BM (oblique arrow) were relatively photopaenic compared to adjacent normal tissues. FDG–PET scanning (not shown) demonstrated no change over the same period.
Enhanced protein synthesis is also an important biological characteristic of malignant tissues. As a proof of principle, C-11 analogues of amino acids have been shown to have enhanced uptake in malignant cells compared to normal tissues. [11C]Methionine has been extensively evaluated and shown to have superior specificity to FDG–PET in some clinical situations. Lack of uptake in the human brain is a major potential advantage of radiolabelled amino acids compared to FDG. Additionally, since the proliferation rate of many brain tumours is relatively low, these agents may also be more sensitive than FLT. As previously discussed, C-11 is not an ideal radioligand for clinical applications and there has been some effort to find suitable fluorinated amino acid analogues. Of potential candidates, [18F]fluoroethyltyrosine (FET) appears a promising candidate. Preliminary studies in brain tumour evaluation appear promising [5].
Both FLT and FET may also have potential advantages with respect to sensitivity of tumour detection relative to FDG in patients with hyperglycaemia or hyperinsulinaemia. As a glucose analogue, FDG uptake into cells is influenced by both insulin and blood glucose levels. Compliance by patients with the requirement for prolonged fasting is variable and inadequate fasting can compromise diagnostic performance of FDG–PET even if blood glucose levels are in the normal range. Despite adequate fasting, diabetic patients may have elevated blood glucose levels that compete directly with FDG, decreasing the sensitivity for lesion detection. Dietary preparation will probably also be a requirement of clinical studies with FET [6] and FLT but will likely not be as significant an impediment to diagnostic results in diabetics.
Since the majority of tumour types that yield false negative FDG–PET results are low-grade carcinomas, FLT is relatively unlikely to provide significant advantages over FDG in such diseases. Alternative tracers are required to image tumours with low FDG-avidity since these cancers can still cause significant morbidity and mortality. Precisely because of their relatively low proliferative rate, they tend to be refractory to conventional chemotherapy agents and to radiotherapy. Many of the tumours known to have variable and sometimes low FDG uptake are low-grade adenocarcinomas. A characteristic of these tumours is high choline content. Assuming that this is also associated with increased choline transport and involved in sterol metabolism, radiolabelled choline analogues have been evaluated as potential PET tracers [7]. [18F]Fluorocholine has shown promise as an imaging agent for providing improved sensitivity for some cancers compared to FDG.
The process of tumour growth and metastasis require development of tumour vascularisation. Inadequate tumour neovascularisation leads to hypoxia and necrosis but through complex pathways may also increase tumour resistance to therapy and malignant potential. There have been several PET tracers developed to image tumour hypoxia in vivo. Most clinical experience is with [18F]fluoromisonidazole (FMISO) [8]. However, other agents have been tested in animals and humans that have some theoretical advantages [9]. Recent experience with a new agent called [18F]fluoroazomycinarabinofuranoside (FAZA) looks promising (Fig. 2) [10].
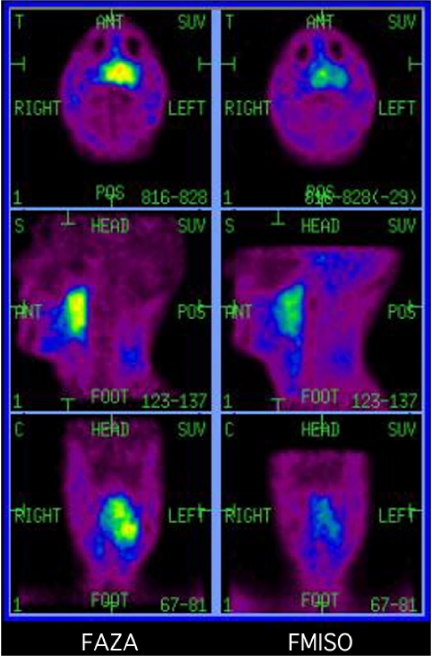
Figure 2.
Most clinical studies of hypoxia imaging have utilized the nitroimadozole, [18F]fluoromisonidazole (FMISO). Slow blood pool clearance and high lipophilicity contribute to significant background activity and relatively low contrast between hypoxic and normal tissues. A new agent [18F]fluoro-azomyacinarabinofuranoside (FAZA) has lower lipophilicity as demonstrated by low brain uptake in the left panel. More rapid blood clearance with similar absolute uptake in hypoxic tissue leads to higher contrast as demonstrated in this comparative study of FAZA (left) and FMISO (right) scans in a patient with locally advanced retropharyngeal cancer. These images are normalised for background activity.
Thus, there is already a range of fluorinated PET tracers that can complement the use of FDG in cancer imaging to enhance either the sensitivity or specificity of disease detection. These agents rely on processes that are fairly generic to malignant transformation and not necessarily specific to any particular cancer. The next generation of cancer imaging PET tracers are those that bind to specific cancer-related receptors or antigens. These agents will likely become important in target identification for molecular therapies.
Conflict of interest
The author has no financial conflict of interest in the publication of this manuscript.
References
- 1.Strauss LG. Fluorine-18-deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23:1409–15. doi: 10.1007/BF01367602. [DOI] [PubMed] [Google Scholar]
- 2.Shields AF, Grierson JR, Dohmen BM , et al. Imaging in vivo proliferation with [18]FLT and positron emission tomography. Nat Med. 1998;11:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 3.Mier W, Haberkorn U, Eisenhut M. [18F]FLT; portrait of a proliferation marker. Eur J Nucl Med. 2002;29:165–9. doi: 10.1007/s00259-001-0675-3. [DOI] [PubMed] [Google Scholar]
- 4.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7. [PubMed] [Google Scholar]
- 5.Weber WA, Wester HJ, Grosu AL , et al. o-(2-[18F]-Fluoroethyl)-l-tyrosine and 1-[methyl-11C] methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27:542–9. doi: 10.1007/s002590050541. [DOI] [PubMed] [Google Scholar]
- 6.Jager PL. Invited commentary: improving amino acid imaging: hungry or stuffed? J Nucl Med. 2002;43:1207–9. [PubMed] [Google Scholar]
- 7.DeGrado TR, Baldwin SW, Wang S, et al. Synthesis and evaluation of 18F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805–14. [PubMed] [Google Scholar]
- 8.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–80. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 9.Chapman JD, Zanzonico P, Ling CC. On measuring hypoxia in individual tumors with radiolabeled agents. J Nucl Med. 2001;42:1653–5. [PubMed] [Google Scholar]
- 10.Sorger D, Patt M, Kumar P, et al. [18F]fluroazomycinarabino- furanoside (18FAZA) and [18F]fluoromisonidazole (18FMISO): a comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl Med Biol. 2003;30:317–26. doi: 10.1016/s0969-8051(02)00442-0. [DOI] [PubMed] [Google Scholar]