Abstract
The subperitoneal space consists of fatty tissue, blood vessels, lymphatics, and lymph nodes enveloped by a serosal lining. This provides a complex interconnecting space which is an important conduit for pathology within the peritoneal cavity. The anatomy and pathology of the subperitoneal space and the surrounding cavity is discussed in its relationship to tumor spread.
Keywords: Peritoneal cavity, subperitoneal spaces, mesentery, omentum
Introduction
Prior to the ready availability of CT it was difficult to directly assess the status of the mesentery, omentum, and peritoneal cavity. These structures were only indirectly assessed by contrast studies of the gastrointestinal tract. An understanding of the intraabdominal spread of disease can only be achieved with a thorough appreciation of the anatomy, including the ligaments and mesenteries, which subdivide the peritoneal cavity.
Embryology of the peritoneal cavity
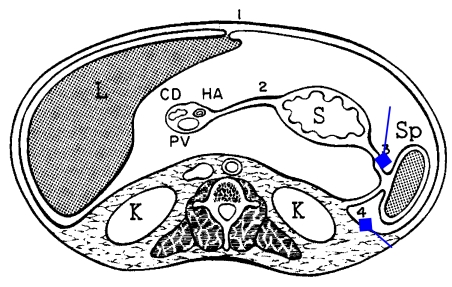
At the embryologic 3-week stage, the primitive gastrointestinal tract is represented by a straight tube supported by a sagittally-oriented mesentery that divides the peritoneal cavity into right and left sides. By the fourth week, the liver begins to develop with the ventral mesentery and the spleen and pancreas within dorsal mesentery. The inferior portion of this mesentery regresses allowing the lower portions of the peritoneal cavity to once again communicate. The tissue anterior to the developing liver, attaching it to the anterior abdominal wall, and containing the umbilical vessels elongates to form the falciform ligament. The tissue between the liver and the stomach becomes the gastrohepatic ligament (GHL) which more inferiorly is the hepatoduodenal ligament (HDL) (Figs. 1 and 2).
Figure 1.
Rotation of peritoneal cavity structure embryologically.
Figure 2.
Embryologic rotation of peritoneal structure with adult relationships.
A period of rapid growth and elongation of the mesenteries occurs with the concurrent rotation. Growth of the liver occurs at the expense of the right peritoneal cavity. With this rotation, the lesser sac, actually a right-sided peritoneal space, develops in the left abdomen and is isolated from the greater peritoneal cavity with the exception of a small communication, the foramen of Winslow. The body of the pancreas develops in the splenorenal ligament (SRL) and should be thought of as an intermesenteric, intraabdominal organ. It subsequently becomes retroperitoneally fused with the dorsal body wall. Incomplete fusion can create a potential intraperitoneal space posterior to the pancreatic tail and, in a similar fashion, one posterior to the pancreatic head. The gastrosplenic ligament (GSL), extending from the stomach inferiorly, becomes elongated and redundant as it extends to the left of the midline finally draping over the transverse colon and small bowel. The layers of the GSL fuse becoming the gastrocolic ligament (GCL). A potential space may remain between these layers as an extension of the lesser sac (omental bursa). The upper GSL fuses with the colon and its dorsal mesentery forming the transverse mesocolon (TMC) [1–3]. The resulting adult mesentery and ligaments represent the remnants of embryologic development and act to compartmentalize intraperitoneal disease processes.
Adult peritoneal spaces, ligaments, and mesenteries
The adult peritoneal cavity can thus be divided into supramesocolic and inframesocolic regions. The more complex supramesocolic space is divided into right and left sides which are further subdivided by upper abdominal organs, ligaments and mesenteries. The inframesocolic space is divided into right and left infracolic regions, paracolic gutters, the pouch of Douglas, and paravesical recesses (Fig. 3).
Figure 3.
Demonstration of lesser sac anatomy. Mitten portion of hand is greater part of lesser sac and thumb represents the superior-recess of the lesser sac.
Right peritoneal space
The right subphrenic space is interposed between the right hemidiaphragm and the liver and limited posteriorly by the bare area. It is continuous anteriorly, as a perihepatic space, with the right subhepatic space (Morison’s pouch) lying along the posterior surface of the right lobe of the liver anterior to the kidney.
The lesser sac (omental bursa), although extending to the left of midline, is a right-sided peritoneal structure. A useful visual aid in understanding lesser sac anatomy has been described by Dodds et al. If one places the right hand across the abdomen with the thumb extended upward and the fingers together over the left upper quadrant the lesser sac can be mimicked. The thumb represents the smaller superior recess and the fingers the larger inferior recess. The inferior recess of the lesser sac between the stomach and spleen is bounded inferiorly by the pancreas and TMC, laterally by the GSL and SRL, and superiorly by the gastrophrenic ligament (GPL). In some cases small bowel loops may displace the TMC superiorly and project into the lesser sac.
Left peritoneal space
The left peritoneal space can be subdivided into four compartments, all of which intercommunicate: left anterior perihepatic space, left posterior perihepatic space (gastrohepatic recess), left anterior subphrenic space, and perisplenic space [4]. The left anterior perihepatic space lies along the surface of the liver and continues posteriorly limited by the bare area of the liver. It is in continuity with the left posterior perihepatic space which is bounded posteriorly by the GPL. It is separated from the lesser sac by the lesser omentum (GHL) and stomach. Anteriorly, perforated ulcers or inflammatory processes of the gallbladder may gain direct access into this space. The left anterior subphrenic space is continuous with the left anterior perihepatic space and is the most common site for an abscess formation after generalized peritonitis. The perisplenic space is a major site for hemorrhagic collections from trauma, post-splenectomy abscess, or collection of fluid from spaces in continuity. The triangular ligament (TL) is seen attaching the liver to the diaphragm.
The inframesocolic space is divided into the smaller right infracolic and the larger left infracolic spaces (LISs) by the small bowel mesentery (SBM). The larger LIS opens into the pelvis and is in continuity with the right paracolic gutter (RPG) which is continuous with the right perihepatic space. This is the dominant pathway of intraabdominal fluid flow. Fluid in the right infracolic space eventually overflows into the left side and gains access to the pelvis. Fluid in the pelvis also tracks along the left paracolic gutter (LPG) as high as the phrenicocolic ligament (PCL). The flow of intraperitoneal fluid is dictated by positioning, respiratory effects, and variations in intraabdominal pressure which favor accumulation in the pelvis, right subhepatic space, and right subphrenic spaces.
Abnormalities of the peritoneal cavity
Fluid collections
Ascites is the most common abnormality of the peritoneal cavity. Fluid tends to distribute itself in the paracolic gutters, subhepatic and subphrenic spaces as well as between leaves of the mesentery. Complex fluid secondary to infection or tumor may be of normal or increased density while acutely hemorrhagic fluid collections are usually of high attenuation. CT is extremely sensitive to even the smallest amounts of fluid, however, there should be caution in interpretation of this finding since ascites may also be seen on delayed enhanced CT scans in normal patients. Delayed enhancement may average 25 HU and occurs in up to 50% of patients with varying pathology. Complex fluid from inflammatory or neoplastic processes should be suspected when there is a significant lesser sac component. The appearance of intraabdominal abscesses is highly variable ranging from fluid to near soft density. Differential diagnosis for fluid collections includes: pseudocysts, chronic hematoma, urinoma, lymphocele, biloma, necrotic neoplasm, complex fluid collections, or even unopacified bowel. The presence of gas is seen in only one-third of abscess cases and may indicate infection, instrumentation, or fistula (Fig. 4(a,b)).
Figure 4.
Sagittal diagrams of left peritoneal space and lesser sac.
Neoplasms
The most common neoplasms involving the peritoneal cavity are metastatic secondary to the ovary, colon, stomach, or pancreas. Tumor may spread along peritoneal surfaces or within ligaments and mesenteries in the subperitoneal space. Peritoneal carcinomatosis is characterized by a number of features: (1) peritoneal thickening and nodules; (2) ascites; (3) loculated ascites; (4) small bowel thickening; and (5) omental disease. Peritoneal implants are best seen when surrounded by ascites, especially at the lateral hepatic margin. In approximately half of the patients the primary tumor can be identified. Peritoneal enhancement may occur in metastatic disease, tuberculous peritonitis, and mesothelioma.
Pseudomyxoma peritonei is a rare complication or rupture of a benign or malignant mucocele of the appendix or mucinous cystadenoma or cystadenocarcinoma of the appendix or ovary. The peritoneal cavity fills with gelatinous material causing scalloping of liver margin and displacement of bowel loops. Septations (which may enhance) often represent the juxtaposed walls of mucinous nodules. Differential diagnosis includes pyogenic peritonitis, lymphoma, or carcinomatosis.
Peritoneal mesothelioma is a rare mesenchymal tumor originating in the serous membranes. Peritoneal primaries account for only 12–20% of all mesothelial neoplasms and may be related to more prolonged asbestos exposure than primary pleural tumors. They spread in sheets along the peritoneal surface causing a rigid pleating of thickened mesenteric leaves. This stellate appearance may also be seen in ovarian, colonic, and pancreatic carcinoma and rarely in lymphomas. Ascites is common but often less prominently seen than in carcinomatosis.
The subperitoneal space as a conduit for disease
The subperitoneal space is a large, interconnecting potential space that extends from the retroperitoneum into the peritoneal cavity [5]. It is primarily composed of fatty tissue with associated nerves, vessels, and lymphatic tissues invested by serosal layers which make up the ligaments and mesenteries associated with the major abdominal organs and viscera. It thus represents a significant conduit for the spread of disease within the peritoneal cavity.
The TMC is a major component of the subperitoneal space. It extends from the pancreas to invest the region of the transverse colon inferiorly. On the right, it is in continuity with the duodenocolic ligament (DCL) and, on the left, the PCL. This structure also has continuity with the GCL representing the greater omentum and extending to the inferior aspect of the stomach. Thus, disease processes, either inflammatory or neoplastic, can spread via the TMC to the colon. Pancreatic disease has a propensity for involving the inferior aspect of the colon while gastric disease, the more superior aspect of the transverse colon.
The GCL, also known as the greater omentum, represents the fused layers of the GSL. It is formed by the fusion of four layers of peritoneum that extend from the stomach and drape over the colon and loops of small bowel extending into the pelvis. Within the fused layers is a variable extension of the inferior aspect of the lesser sac termed the omental bursa. The greater omentum is saturated with phagocytic cells and attempts to ingest inflammatory as well as neoplastic disease. It thus can be easily involved with tumor or infection and also is used for its fat in various surgical procedures.
The GHL is represented by the lesser omentum (LO) and extends from the liver to the stomach. It contains the left gastric artery, coronary vein, and left gastric nodes. Gastric neoplasms may spread easily within this ligamentous structure and can be seen as soft tissue nodes surrounded by the adipose tissue within the layers of the ligamentous structure. Gastric malignancy can spread into the left lobe of the liver via this ligament which continues into the liver as Glisson’s capsule.
The HDL is the free edge of the GHL and extends to the anterior pararenal space on the right. It represents a pathway of spread of pancreaticobiliary disease.
The GSL is the left lateral extension of the greater omentum connecting the greater curvature of the stomach with the spleen. Within this ligament lie the gastroepiploic and short gastric vessels. It is a conduit for the spread of disease between the pancreatic tail, spleen, and stomach.
The SRL surrounds a region of the pancreatic tail and extends to the left anterior pararenal space. This most commonly represents a conduit of disease from the pancreas to the retroperitoneum.
The PCL, the left margin of the TMC, is a suspensory ligament of the spleen and reflects along the anatomic splenic flexure of the colon. It acts as a conduit among the pancreas, colon, spleen, and left retroperitoneum for the spread of disease.
The SBM is a vast structure within the peritoneal cavity. It is best appreciated as an abundant fatty structure which shows numerous vessels coursing through it. Nodes in the mesentery should be less than 1 cm. This is frequently involved with lymphoma. Processes may also spread hematogenously or along the surface of the leaves of the mesentery. In the case of peritoneal mesothelioma, there is a thickening between the leaves of the mesentery and soft tissue density can be seen separating these relatively homogeneous fatty leaflets.
The colonic mesentery (CM) is much less abundant than the SBM. It is generally short in the area of the ascending and descending colons where it acts as a suspensory ligament. It is more well-developed in the area of the TMC and the sigmoid mesocolon. Various processes involving the sigmoid colon including diverticulitis and colonic malignancy can involve this structure. It also may be involved with metastatic disease from pelvic malignancies, especially ovarian carcinoma.
Mesentery and omentum
The SBM is a fan-shaped structure extending obliquely from the duodenojejunal function to the ileocecal valve. Portions of the colon are also supported by the mesenteries [6]. The mesentery consists of a network of spaces covered by mesothelium enclosing fat, blood vessels, lymphatic channels and nodes. Mesenteric pathology appears in a spectrum: rounded or ‘cake-like’ masses, ill-defined masses, or a stellate appearance. Rounded or cake-like masses are usually secondary to metastases or lymphoma.
Benign desmoid tumors are rare. These appear as soft tissue masses. Mesenteric cysts are also rare and are characterized by their low attenuation. These may be duplication cysts, lymphangiomas, non-pancreatic pseudocysts, mesothelial cysts, enteric cysts, or duplication cysts.
Ill-defined masses are often secondary to neoplasm, hematoma, or inflammatory conditions. Inflammatory processes are most frequently the result of adjacent disease including pancreatitis, inflammatory bowel disease, and diverticulitis. Mesenteric panniculitis or retractile mesenteritis is an unusual, non-specific, non-malignant primary inflammatory process. Abdominal involvement in tuberculous peritonitis is rare and usually occurs in patients with underlying risk factors: intravenous drug abuse, alcoholism, AIDS, and steroid usage. Resulting CT features consist of: adenopathy (often low-attenuation), preferentially mesenteric or peripancreatic often in close proximity to involved portions of the gastrointestinal tract. Carcinoid tumors present as solid masses with ill-defined margins characterized by radiating linear areas of fibrosis. Mesenteric edema is a benign metabolic condition which causes a diffuse characteristic increase in density of the mesenteric fat and indistinctness of the margins of mesenteric vessels. Bowel wall thickening may be present and is most often secondary to hypoalbuminemia. Mesenteric venous thrombosis is characterized by enlargement of the vein that is low density and surrounded by an enhancing wall.
Omental pathology most often appears as nodular or solid masses—‘omental caking’—when secondary to metastatic disease [7, 8]. A micronodular, finely infiltrated, smudged appearance may be seen with metastases, mesothelioma, or tuberculosis. Cystic omental lesions are usually the result of primary cysts, pancreatitis, abscesses, necrotic tumor, or hematoma.
Summary
CT is an exquisite method for defining pathology involving the peritoneal cavity, mesentery, and omentum. A thorough knowledge of the embryology and anatomy assists in understanding the distribution of disease processes. The characteristic appearance of many lesions assists in differential diagnosis.
References
- 1.Dodds WJ, Foley WD, Lawson TL, Stewart ET, Taylor A. Anatomy and imaging of the lesser peritoneal sac. Am J Roentgenol. 1985;144:567–75. doi: 10.2214/ajr.144.3.567. [DOI] [PubMed] [Google Scholar]
- 2.Meyers MA. Dynamic Radiology of the Abdomen: Normal and Pathologic Anatomy. 2nd edn. New York: Springer-Verlag; 1982. [Google Scholar]
- 3.Meyers MA, Oliphant M, Berne AS, Feldberg MA. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593–604. doi: 10.1148/radiology.163.3.3575702. [DOI] [PubMed] [Google Scholar]
- 4.Gore RM, Meyers MA. Pathways of abdominal and pelvic disease spread. In: Gore RM, Levine MS, editors. Gastrointestinal Radiology. 2nd edn., vol. 2. Philadelphia, PA: W.B. Saunders; 2000. pp. 1948–68. [Google Scholar]
- 5.Oliphant M, Berne AS. Computed tomography of the subperitoneal space: demonstration of direct spread on intraabdominal disease. J Comput Assist Tomogr. 1982;6:1127–37. doi: 10.1097/00004728-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Silverman PM, Kelvin FM, Korobkin M, Dunnick NR. Computed tomography of the normal mesentery. Am J Roentgenol. 1984;143:953–7. doi: 10.2214/ajr.143.5.953. [DOI] [PubMed] [Google Scholar]
- 7.Silverman PM, Cooper C. Mesenteric and omental lesions. In: Gore RM, Levine MS, editors. Gastrointestinal Radiology. 2nd edn., vol. 2. Philadelphia, PA: W.B. Saunders; 2000. pp. 1980–92. [Google Scholar]
- 8.Cooper C, Jeffrey RB, Silverman PM, Federle MP, Chun GH. Computed tomography of omental pathology. J Comput Assist Tomogr. 1986;10:62–6. doi: 10.1097/00004728-198601000-00013. [DOI] [PubMed] [Google Scholar]