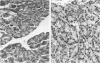
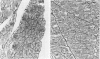
Abstract
Surgical diversion of bile and pancreatic secretions to the mid small bowel has been shown to provoke increased CCK plasma concentration and growth of the pancreas in rats. This study was undertaken to investigate the effects of chronic pancreaticobiliary diversion on pancreatic morphology as well as the circulating concentrations of pancreatic polypeptide, secretin, gastrin, and CCK. Fifteen month diversion provoked 73 and 86% increases in pancreatic weight and volume (p less than 0.001). Cholecystokinin blood concentration increased by 98%, from 20.9 +/- 5.7 pg/ml in controls to 41.3 +/- 5.4 after diversion (p less than 0.05), but pancreatic polypeptide, secretin, and gastrin levels were not affected. The volume of the exocrine pancreas doubled from 1104.6 +/- 78.2 mm3 in controls to 2201.2 +/- 229.2 (p less than 0.001), with a matching increase in interstitial tissue. On the contrary, the volume of the endocrine pancreas remained unchanged. Hyperplastic nodules developed in the exocrine pancreas, in 71% of diverted rats, but not in transected controls. We conclude from these observations that chronic diversion of bile and pancreatic juice stimulated pancreatic growth, most likely through a persistent rise of CCK plasma concentrations. Furthermore, this long lasting stimulation induced the development of exocrine pancreatic nodules.
Full text
PDF

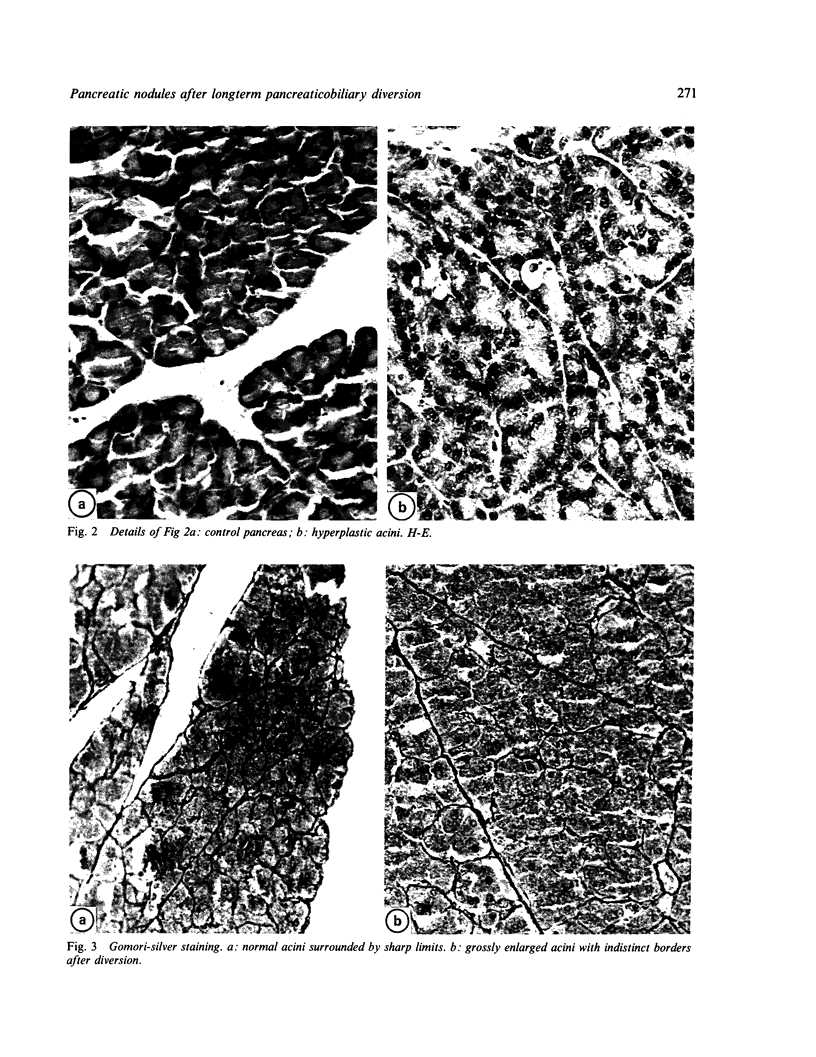
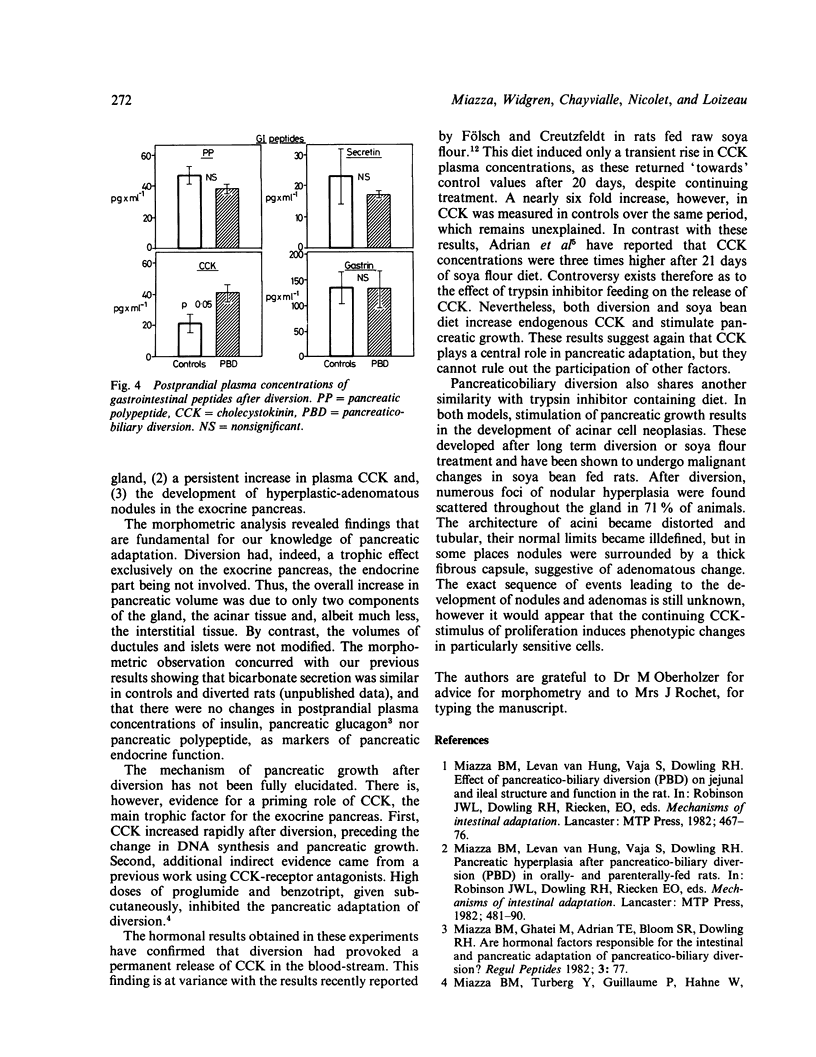

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fölsch U. R., Creutzfeldt W. Adaptation of the pancreas during treatment with enzyme inhibitors in rats and man. Scand J Gastroenterol Suppl. 1985;112:54–63. doi: 10.3109/00365528509092213. [DOI] [PubMed] [Google Scholar]
- McGuinness E. E., Morgan R. G., Levison D. A., Hopwood D., Wormsley K. G. Interaction of azaserine and raw soya flour on the rat pancreas. Scand J Gastroenterol. 1981;16(1):49–56. [PubMed] [Google Scholar]
- Miazza B. M., Turberg Y., Guillaume P., Hahne W., Chayvialle J. A., Loizeau E. Mechanism of pancreatic growth induced by pancreatico-biliary diversion in the rat. Inhibition by proglumide, benzotript, and ranitidine. Scand J Gastroenterol Suppl. 1985;112:75–83. doi: 10.3109/00365528509092216. [DOI] [PubMed] [Google Scholar]
- Miazza B., Palma R., Lachance J. R., Chayvialle J. A., Jonard P. P., Modigliani R. Jejunal secretory effect of intraduodenal food in humans. A comparison of mixed nutrients, proteins, lipids, and carbohydrates. Gastroenterology. 1985 May;88(5 Pt 1):1215–1222. doi: 10.1016/s0016-5085(85)80082-2. [DOI] [PubMed] [Google Scholar]
- Pelletier M. J., Chayvialle J. A., Minaire Y. Uneven and transient secretin release after a liquid test meal. Gastroenterology. 1978 Dec;75(6):1124–1132. [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]