Abstract
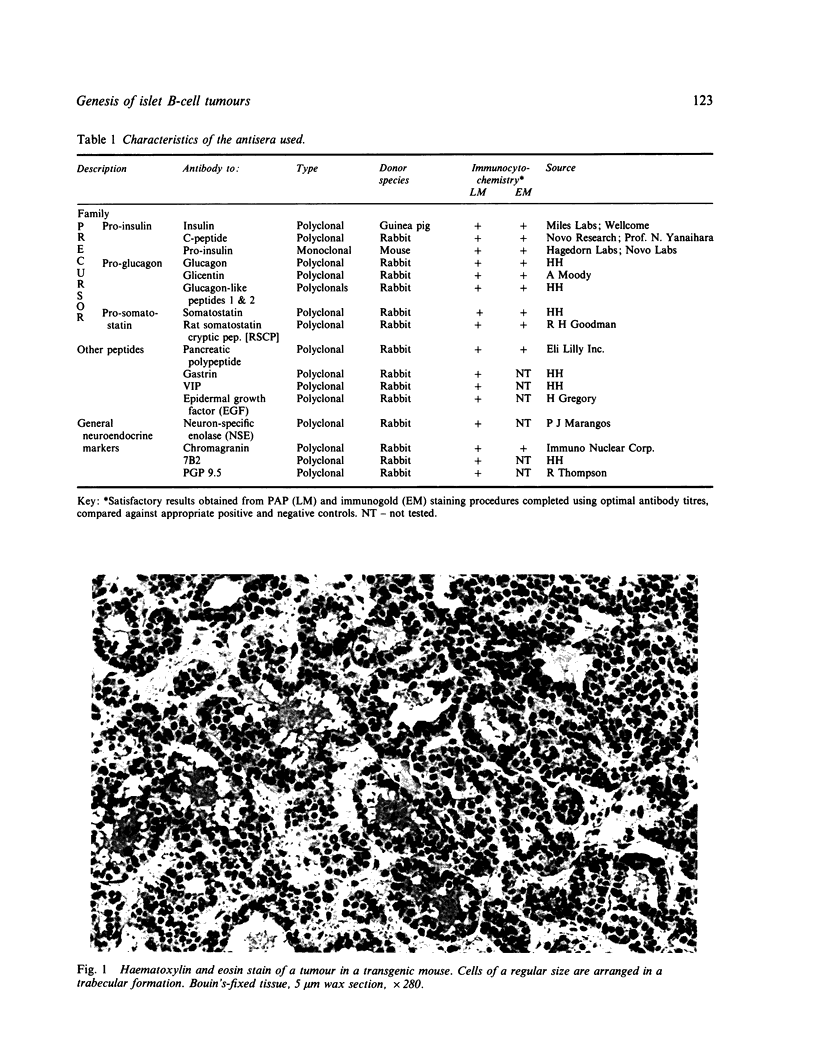
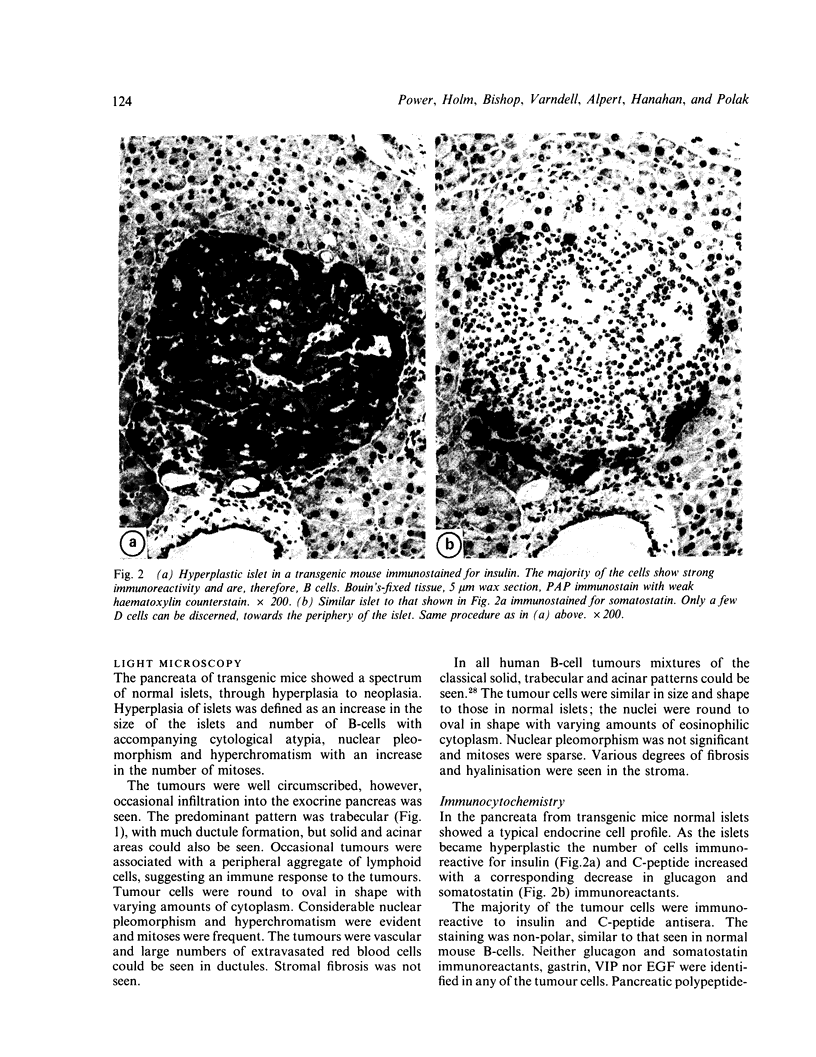
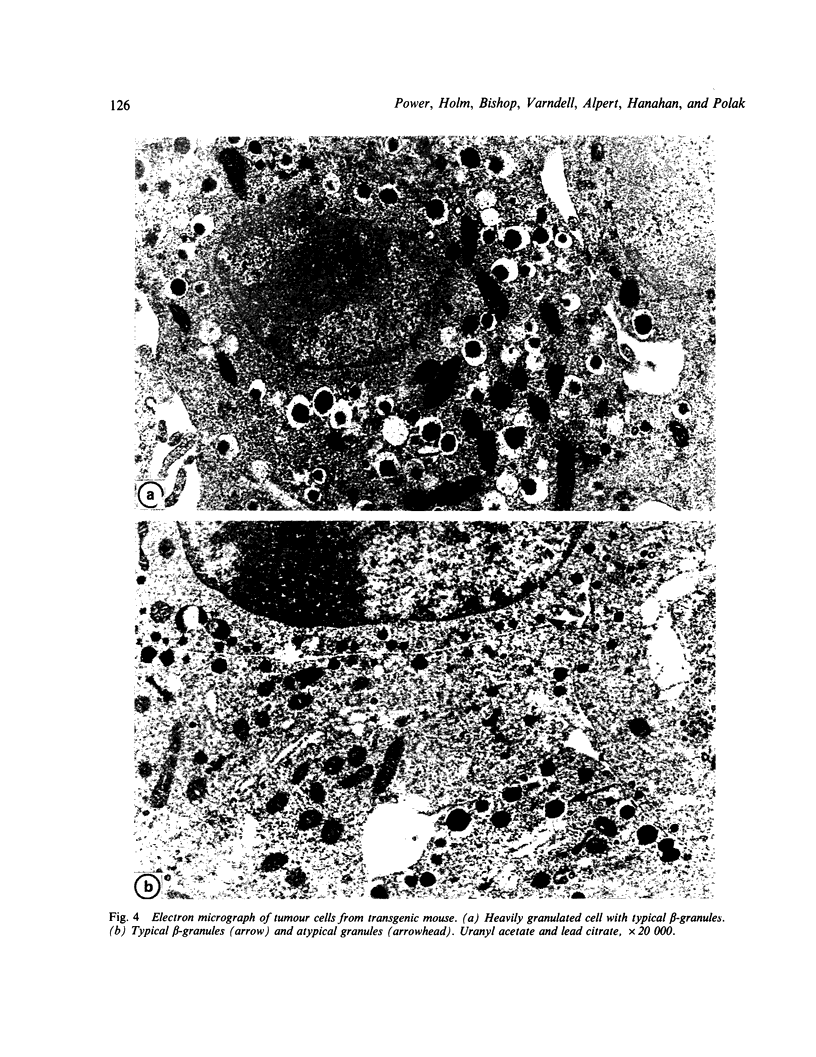
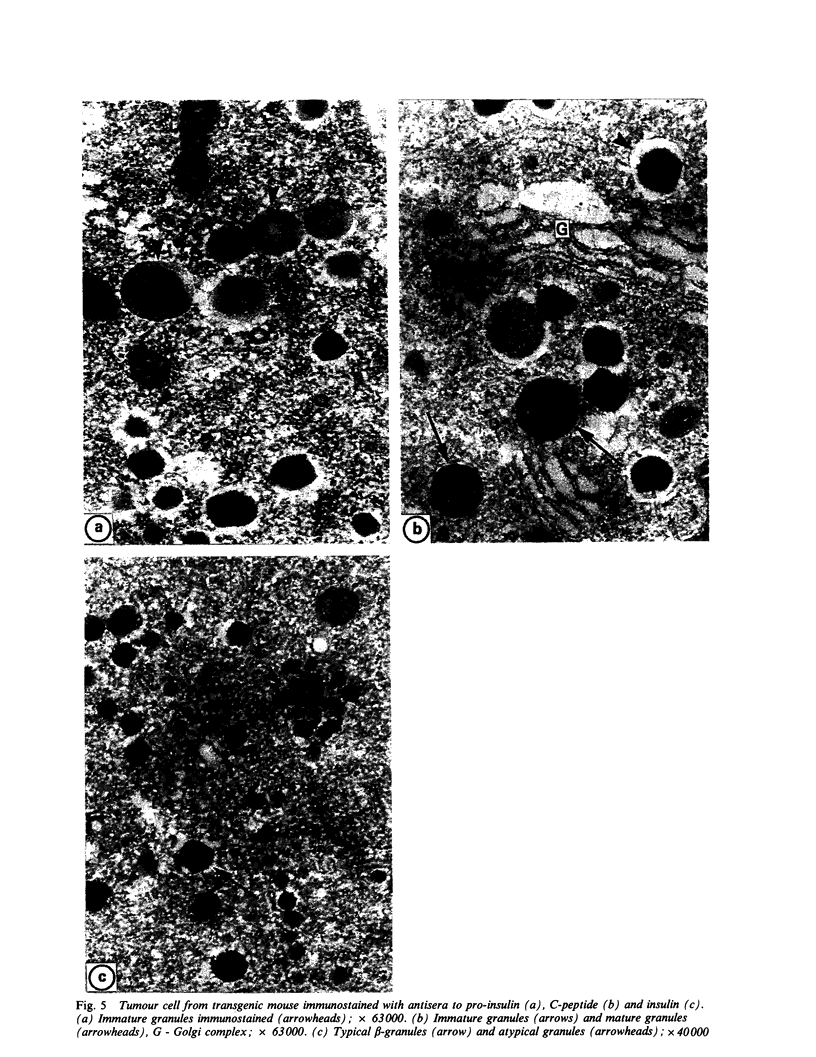
The transformation and adaptation of pancreatic insulin-producing (B) cells has been studied in a transgenic mouse model using a panel of antisera recognising peptides and general neuroendocrine markers at both light and electron microscopical levels. Stages of tumour genesis in the transgenic mouse model from hyperplasia to neoplasia, have been compared with human B-cell tumours. A normal complement of peptide containing cells was seen in the transgenic mouse pancreas, but cells containing pro-insulin-derived peptides became more numerous as hyperplasia commenced. The transgenic mouse tumours were composed of B cells, although 30-35% of the tumours were also found to contain PP cells--a finding which is directly comparable with that in human insulin-producing tumours. NSE, 7B2 and chromogranin immunoreactivities were found in most cells from all the tumours examined. Antisera to PGP 9.5, a novel marker for elements of the neuroendocrine system, were found to stain hyperplastic and neoplastic B-cells intensely. In contrast, normal mouse B-cells did not show PGP 9.5 immunoreactivity thus it appears that PGP 9.5 is differentially expressed in transformed and/or growing mouse B-cells and hence may be used as an indicator in studies of early tumour growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Facer P., Ferri G. L., Marangos P. J., Pearse A. G. Neuron specific enolase: a common marker for the endocrine cells and innervation of the gut and pancreas. Gastroenterology. 1982 Oct;83(4):902–915. [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bradbury J. M., Thompson R. J. Immunoassay of the neuronal and neuroendocrine marker PGP 9.5 in human tissues. J Neurochem. 1985 Feb;44(2):651–653. doi: 10.1111/j.1471-4159.1985.tb05461.x. [DOI] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Doran J. F., Jackson P., Kynoch P. A., Thompson R. J. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983 Jun;40(6):1542–1547. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimelius L. The argyrophil reaction in islet cells of adult human pancreas studies with a new silver nitrate procedure. Acta Soc Med Ups. 1968;73(5-6):271–294. [PubMed] [Google Scholar]
- Gulbenkian S., Wharton J., Polak J. M. The visualisation of cardiovascular innervation in the guinea pig using an antiserum to protein gene product 9.5 (PGP 9.5). J Auton Nerv Syst. 1987 Mar;18(3):235–247. doi: 10.1016/0165-1838(87)90122-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Oncogenes in transgenic mice. Nature. 1984 Dec 6;312(5994):503–504. doi: 10.1038/312503a0. [DOI] [PubMed] [Google Scholar]
- Heitz P. U., Kasper M., Polak J. M., Klöppel G. Pancreatic endocrine tumors. Hum Pathol. 1982 Mar;13(3):263–271. doi: 10.1016/s0046-8177(82)80183-4. [DOI] [PubMed] [Google Scholar]
- Hsi K. L., Seidah N. G., De Serres G., Chrétien M. Isolation and NH2-terminal sequence of a novel porcine anterior pituitary polypeptide. Homology to proinsulin, secretin and Rous sarcoma virus transforming protein TVFV60. FEBS Lett. 1982 Oct 18;147(2):261–266. doi: 10.1016/0014-5793(82)81055-7. [DOI] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Kirshner A. G., Kirshner N. A specific soluble protein from the catecholamine storage vesicles of bovine adrenal medulla. II. Physical characterization. Biochim Biophys Acta. 1969 May;181(1):219–225. doi: 10.1016/0005-2795(69)90244-x. [DOI] [PubMed] [Google Scholar]
- Liu T. H., Tseng H. C., Zhu Y., Zhong S. X., Chen J., Cui Q. C. Insulinoma. An immunocytochemical and morphologic analysis of 95 cases. Cancer. 1985 Sep 15;56(6):1420–1429. doi: 10.1002/1097-0142(19850915)56:6<1420::aid-cncr2820560633>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Zomzely-Neurath C., York C. Determination and characterization of neuron specific protein (NSP) associated enolase activity. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1309–1316. doi: 10.1016/0006-291x(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., Evans R. M. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982 Dec 16;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Adrian T. E., Heitz P., Bryant M. G., Pearse A. G. Pancreatic polypeptide in insulinomas, gastrinomas, vipomas, and glucagonomas. Lancet. 1976 Feb 14;1(7955):328–330. doi: 10.1016/s0140-6736(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978 Dec 21;276(5690):834–836. doi: 10.1038/276834a0. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Hsi K. L., De Serres G., Rochemont J., Hamelin J., Antakly T., Cantin M., Chrétien M. Isolation and NH2-terminal sequence of a highly conserved human and porcine pituitary protein belonging to a new superfamily. Immunocytochemical localization in pars distalis and pars nervosa of the pituitary and in the supraoptic nucleus of the hypothalamus. Arch Biochem Biophys. 1983 Sep;225(2):525–534. doi: 10.1016/0003-9861(83)90063-2. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Ghatei M. A., Williams S. J., Uttenthal L. O., Facer P., Bishop A. E., Polak J. M., Bloom S. R. Production of pituitary protein 7B2 immunoreactivity by endocrine tumors and its possible diagnostic value. J Clin Endocrinol Metab. 1986 Sep;63(3):758–765. doi: 10.1210/jcem-63-3-758. [DOI] [PubMed] [Google Scholar]
- Thompson R. J., Doran J. F., Jackson P., Dhillon A. P., Rode J. PGP 9.5--a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983 Nov 14;278(1-2):224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Underwood L. E., D'Ercole A. J. Insulin and insulin-like growth factors/somatomedins in fetal and neonatal development. Clin Endocrinol Metab. 1984 Mar;13(1):69–89. doi: 10.1016/s0300-595x(84)80009-2. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]