Abstract
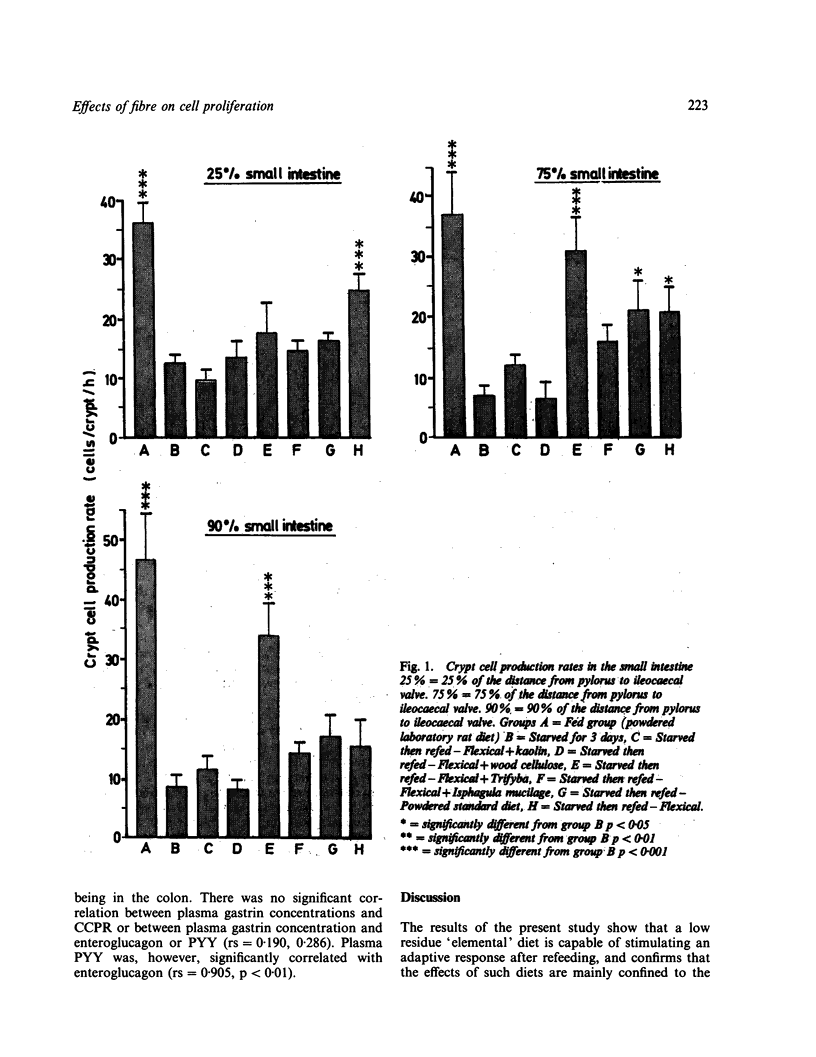
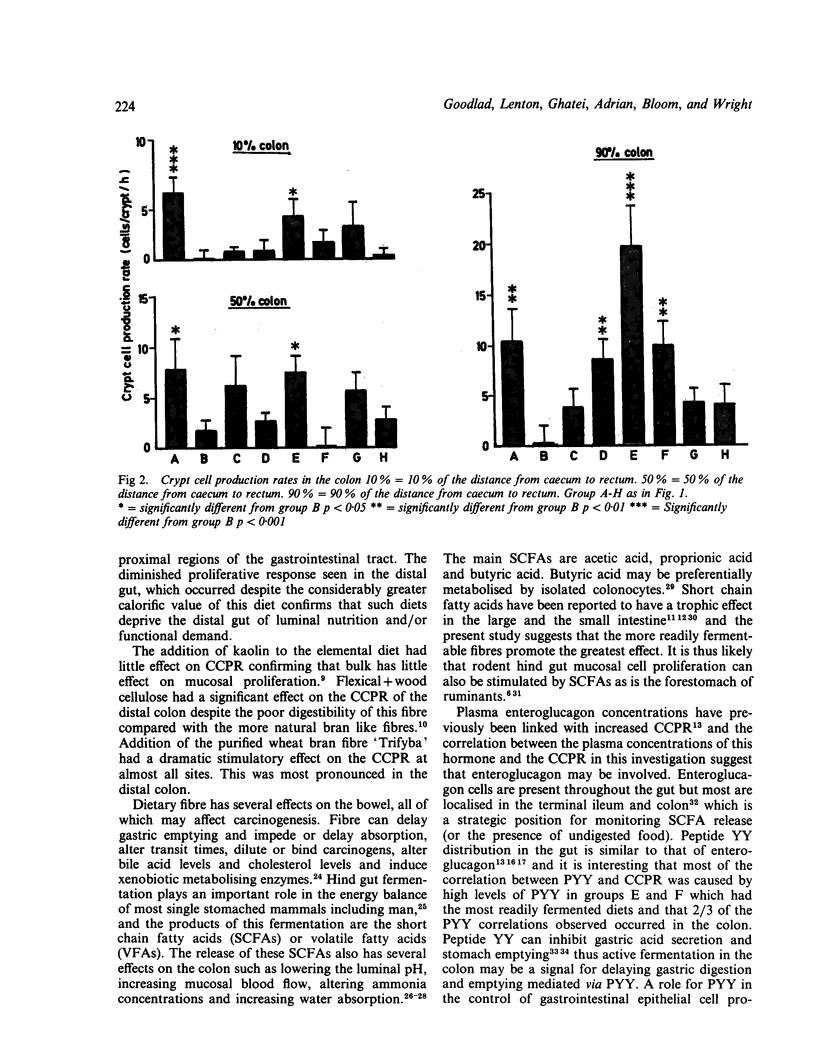
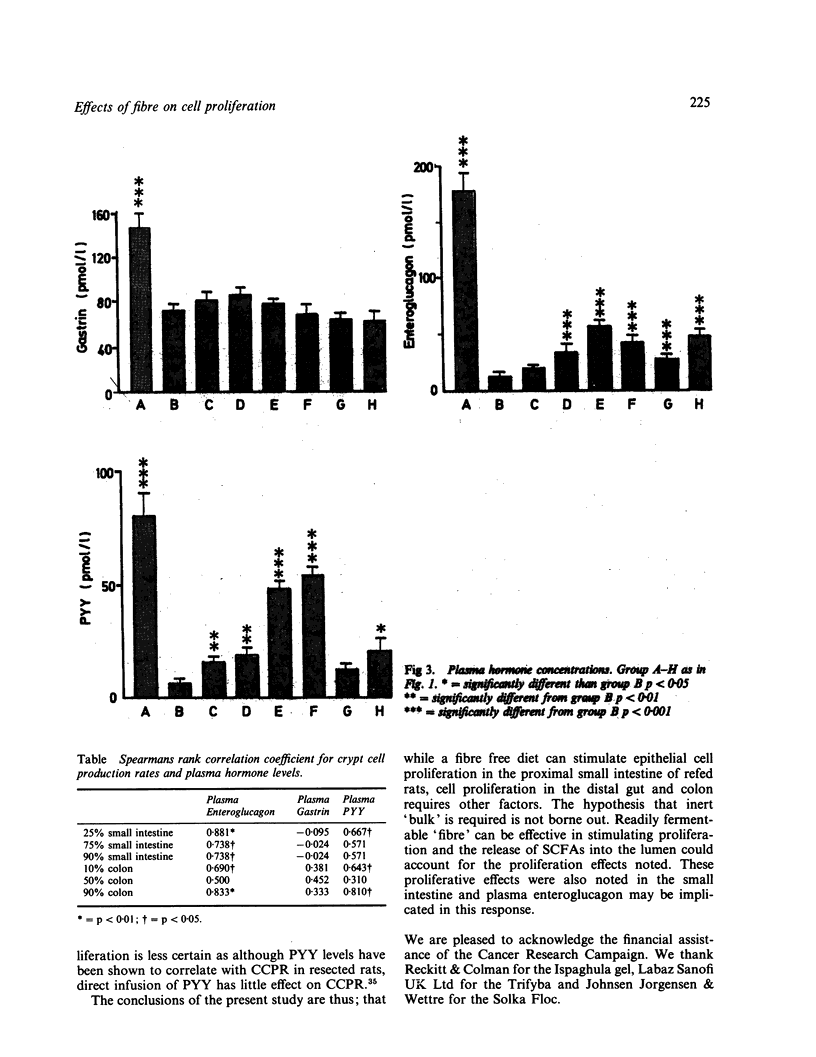
Refeeding starved rats with a fibre free 'elemental' diet increased crypt cell production rate (CCPR) in the proximal small intestine but not in the distal regions of the gut. Little effect on CCPR was seen when inert bulk (kaolin) was added to the 'elemental' diet. Addition of a poorly fermentable dietary 'fibre' (purified wood cellulose) had little effect on intestinal epithelial cell proliferation except in the distal colon where it significantly increased CCPR. A more readily fermentable 'fibre' (purified wheat bran) caused a large proliferative response in the proximal, mid and distal colon and in the distal small intestine. A gel forming 'fibre' also stimulated proliferation in the distal colon. There was no significant correlation between CCPR and plasma gastrin concentrations, but plasma enteroglucagon concentrations were significantly correlated with CCPR in almost all the sites studied. Plasma PYY concentrations also showed some correlation with CCPR, especially in the colon. Thus, whilst inert bulk cannot stimulate colonic epithelial cell proliferation, fermentable 'fibre' is capable of stimulating proliferation in the colon, and especially in the distal colon: it can also stimulate proliferation in the distal small intestine and it is likely that plasma enteroglucagon may have a role to play in this process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Ferri G. L., Bacarese-Hamilton A. J., Fuessl H. S., Polak J. M., Bloom S. R. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985 Nov;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Alford F. P., Bloodm S. R., Nabarro J. D. Glucagon levels in normal and diabetic subjects: use of a specific immunoabsorbent for glucagon radioimmunoassay. Diabetologia. 1977 Jan;13(1):1–6. doi: 10.1007/BF00996319. [DOI] [PubMed] [Google Scholar]
- Ali-Rachedi A., Varndell I. M., Adrian T. E., Gapp D. A., Van Noorden S., Bloom S. R., Polak J. M. Peptide YY (PYY) immunoreactivity is co-stored with glucagon-related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry. 1984;80(5):487–491. doi: 10.1007/BF00495439. [DOI] [PubMed] [Google Scholar]
- Allen J. M., Fitzpatrick M. L., Yeats J. C., Darcy K., Adrian T. E., Bloom S. R. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion. 1984;30(4):255–262. doi: 10.1159/000199117. [DOI] [PubMed] [Google Scholar]
- Bloom S. R. Gut and brain--endocrine connections. The Goulstonian Lecture 1979. J R Coll Physicians Lond. 1980 Jan;14(1):51–57. [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Cellulose and the human gut. Gut. 1984 Aug;25(8):805–810. doi: 10.1136/gut.25.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Short chain fatty acids in the human colon. Gut. 1981 Sep;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M., Grimelius L., Wilander E., Ryberg B., Terenius L., Lundberg J. M., Tatemoto K. Immunocytochemical identification of polypeptide YY (PYY) cells in the human gastrointestinal tract. Histochemistry. 1983;77(1):15–23. doi: 10.1007/BF00496632. [DOI] [PubMed] [Google Scholar]
- El-Salhy M., Wilander E., Juntti-Berggren L., Grimelius L. The distribution and ontogeny of polypeptide YY (PYY)- and pancreatic polypeptide (PP)-immunoreactive cells in the gastrointestinal tract of rat. Histochemistry. 1983;78(1):53–60. doi: 10.1007/BF00491111. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Wright N. A. Effects of addition of kaolin or cellulose to an elemental diet on intestinal cell proliferation in the mouse. Br J Nutr. 1983 Jul;50(1):91–98. doi: 10.1079/bjn19830075. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R., Lupton J. R. Effect of dietary fibers on rat large bowel mucosal growth and cell proliferation. Am J Physiol. 1984 Apr;246(4 Pt 1):G378–G385. doi: 10.1152/ajpgi.1984.246.4.G378. [DOI] [PubMed] [Google Scholar]
- Janne P., Carpentier Y., Willems G. Colonic mucosal atrophy induced by a liquid elemental diet in rats. Am J Dig Dis. 1977 Sep;22(9):808–812. doi: 10.1007/BF01694512. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Granger D. N. Effect of volatile fatty acids on blood flow and oxygen uptake by the dog colon. Gastroenterology. 1981 May;80(5 Pt 1):962–969. [PubMed] [Google Scholar]
- Lehnert S. Changes in growth kinetics of jejunal epithelium in mice maintained on an elemental diet. Cell Tissue Kinet. 1979 May;12(3):239–248. doi: 10.1111/j.1365-2184.1979.tb00146.x. [DOI] [PubMed] [Google Scholar]
- McNeil N. I. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984 Feb;39(2):338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- Morin C. L., Ling V., Bourassa D. Small intestinal and colonic changes induced by a chemically defined diet. Dig Dis Sci. 1980 Feb;25(2):123–128. doi: 10.1007/BF01308310. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Russel R. C., Bloom S. R., Fielding L. P., Bryant M. G. Current problems in the measurement of gastrin release. A reproducible measure of physiological gastrin release. Postgrad Med J. 1976 Oct;52(612):645–650. doi: 10.1136/pgmj.52.612.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. P., Dudrick S. J., Copeland E. M., Johnson L. R. Effects of various diets on colonic growth in rats. Gastroenterology. 1979 Oct;77(4 Pt 1):658–663. [PubMed] [Google Scholar]
- Sakata T., Yajima T. Influence of short chain fatty acids on the epithelial cell division of digestive tract. Q J Exp Physiol. 1984 Jul;69(3):639–648. doi: 10.1113/expphysiol.1984.sp002850. [DOI] [PubMed] [Google Scholar]
- Savage A. P., Gornacz G. E., Adrian T. E., Ghatei M. A., Goodlad R. A., Wright N. A., Bloom S. R. Is raised plasma peptide YY after intestinal resection in the rat responsible for the trophic response? Gut. 1985 Dec;26(12):1353–1358. doi: 10.1136/gut.26.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storme G., Willems G. The effect of a liquid elemental diet on cell proliferation in the colon of rats. Cell Tissue Res. 1981;216(1):221–225. doi: 10.1007/BF00234557. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Nakaya M., Itoh Z., Tatemoto K., Mutt V. Inhibition of interdigestive contractile activity in the stomach by peptide YY in Heidenhain pouch dogs. Gastroenterology. 1983 Jul;85(1):114–121. [PubMed] [Google Scholar]
- Tasman-Jones C., Owen R. L., Jones A. L. Semipurified dietary fiber and small-bowel morphology in rats. Dig Dis Sci. 1982 Jun;27(6):519–524. doi: 10.1007/BF01296731. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980 Jun 5;285(5764):417–418. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]
- Tutton P. J., Barkla D. H. Further studies on the effect of adenosine cyclic monophosphate derivatives on cell proliferation in the jejunal crypts of rat. Clin Exp Pharmacol Physiol. 1982 Nov-Dec;9(6):671–674. doi: 10.1111/j.1440-1681.1982.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Van Soest P. J. Some physical characteristics of dietary fibres and their influence on the microbial ecology of the human colon. Proc Nutr Soc. 1984 Jan;43(1):25–33. doi: 10.1079/pns19840024. [DOI] [PubMed] [Google Scholar]


