Abstract
The adaptive changes of hypertrophy and hyperplasia are diffuse and reversible responses of the pancreas to growth promoting stimuli. Early stages of neoplastic growth in the pancreas have been studied in carcinogen treated animals and preneoplastic lesions including atypical acinar cell foci and nodules, tubular ductal complexes and intraductal hyperplasia were identified. Neoplastic growth is clonal rather than diffuse and involves multiple steps through preneoplastic stages to produce a tumour. The individual steps are commonly regarded as reflecting a series of changes in the genome of the cells. Although the changes are likely to be irreversible, completion of the sequence usually requires a major portion of the lifespan of the host. The rate of progression of preneoplastic lesions to cancer may be modulated by the same factors that control adaptive growth. It follows that such factors will influence the probability that a carcinoma will develop. Cholecystokinin (CCK) seems to provide one example of a hormone/growth factor that can stimulate normal, adaptive, and neoplastic growth, and it is to be expected that other such hormones will be identified.
Full text
PDF

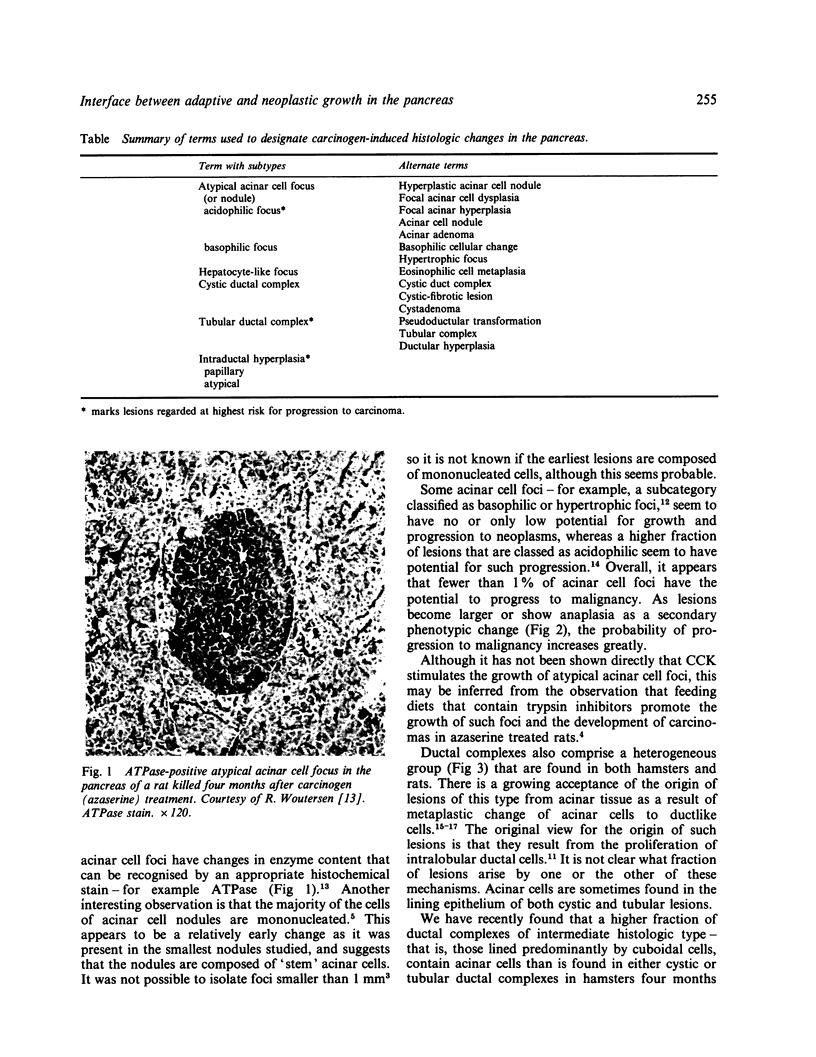
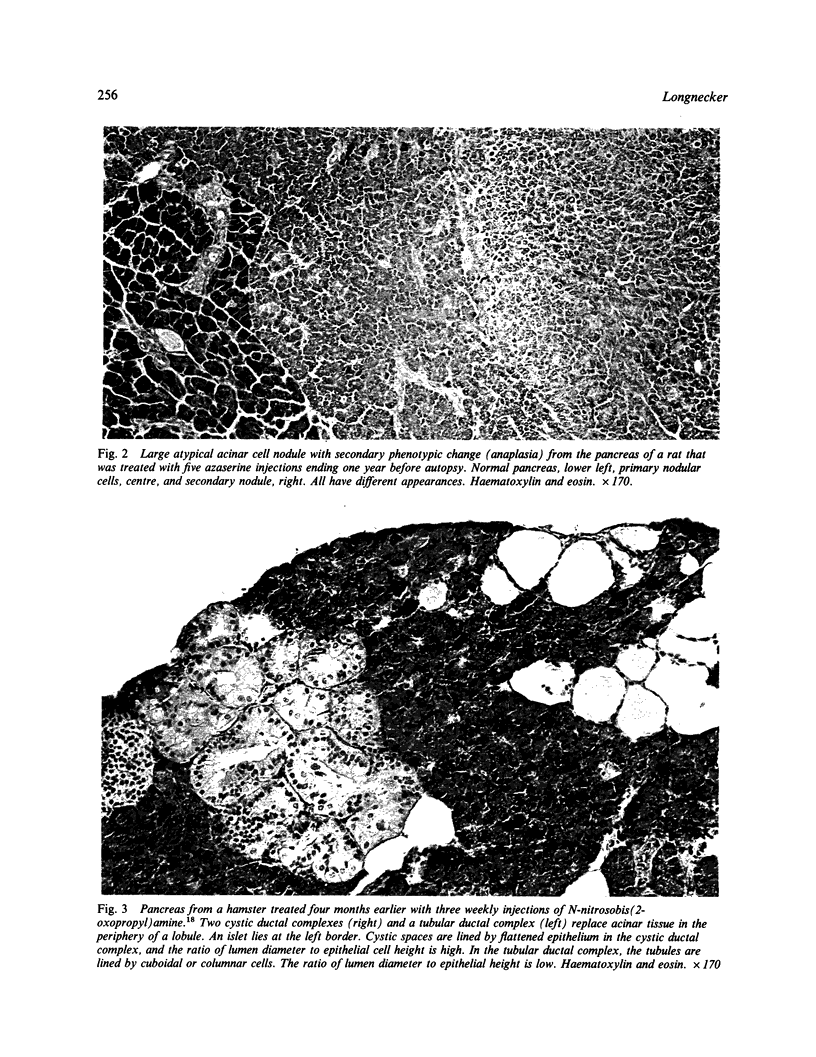


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax J., Feringa A. W., van Garderen-Hoetmer A., Woutersen R. A., Scherer E. Adenosine triphosphatase, a new marker for the differentiation of putative precancerous foci induced in rat pancreas by azaserine. Carcinogenesis. 1986 Mar;7(3):457–462. doi: 10.1093/carcin/7.3.457. [DOI] [PubMed] [Google Scholar]
- Chiu T. Hypertrophic foci of pancreatic acinar cells in rats. Crit Rev Toxicol. 1985;14(2):133–157. doi: 10.3109/10408448509089852. [DOI] [PubMed] [Google Scholar]
- Flaks B. Histogenesis of pancreatic carcinogenesis in the hamster: ultrastructural evidence. Environ Health Perspect. 1984 Jun;56:187–203. doi: 10.1289/ehp.8456187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman W. P., Jansen J. B., Lamers C. B. Plasma cholecystokinin response to oral fat in patients with Billroth I and Billroth II gastrectomy. Ann Surg. 1984 Mar;199(3):276–280. doi: 10.1097/00000658-198403000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson A. G., Carter D. C. Pancreatic carcinogenesis-enhancement by cholecystokinin in the hamster-nitrosamine model. Br J Cancer. 1985 Jan;51(1):107–114. doi: 10.1038/bjc.1985.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker D. S., French J., Hyde E., Lilja H. S., Yager J. D., Jr Effect of age on nodule induction by azaserine and DNA synthesis in rat pancreas. J Natl Cancer Inst. 1977 Jun;58(6):1769–1775. doi: 10.1093/jnci/58.6.1769. [DOI] [PubMed] [Google Scholar]
- Longnecker D. S., Wiebkin P., Schaeffer B. K., Roebuck B. D. Experimental carcinogenesis in the pancreas. Int Rev Exp Pathol. 1984;26:177–229. [PubMed] [Google Scholar]
- Mack T. M., Yu M. C., Hanisch R., Henderson B. E. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst. 1986 Jan;76(1):49–60. [PubMed] [Google Scholar]
- Morgan R. G., Schaeffer B. K., Longnecker D. S. Size and number of nuclei differ in normal and neoplastic acinar cells from rat pancreas. Pancreas. 1986;1(1):37–43. doi: 10.1097/00006676-198601000-00008. [DOI] [PubMed] [Google Scholar]
- Oates P. S., Morgan R. G. Changes in pancreatic acinar cell nuclear number and DNA content during aging in the rat. Am J Anat. 1986 Dec;177(4):547–554. doi: 10.1002/aja.1001770413. [DOI] [PubMed] [Google Scholar]
- Pictet R. L., Clark W. R., Williams R. H., Rutter W. J. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972 Dec;29(4):436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Subbarao V., Luetteke N., Scarpelli D. G. Further characterization of carcinogen-induced hepatocytelike cells in hamster pancreas. Am J Pathol. 1983 Jan;110(1):89–94. [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Upton M. P., Subbarao V., Scarpelli D. G. Two populations of cells with differing proliferative capacities in atypical acinar cell foci induced by 4-hydroxyaminoquinoline-1-oxide in the rat pancreas. Lab Invest. 1982 May;46(5):527–534. [PubMed] [Google Scholar]
- Scherer E. Neoplastic progression in experimental hepatocarcinogenesis. Biochim Biophys Acta. 1984;738(4):219–236. doi: 10.1016/0304-419x(83)90005-7. [DOI] [PubMed] [Google Scholar]
- Woutersen R. A., van Garderen-Hoetmer A., Longnecker D. S. Characterization of a 4-month protocol for the quantitation of BOP-induced lesions in hamster pancreas and its application in studying the effect of dietary fat. Carcinogenesis. 1987 Jun;8(6):833–837. doi: 10.1093/carcin/8.6.833. [DOI] [PubMed] [Google Scholar]






