Abstract
Local, regional and distant tumor recurrence is common following surgical resection for non-small cell lung cancer. It is important to be familiar with the patterns of recurrence and to differentiate them from the normal post-operative appearance and post-radiation changes. The risks and types of recurrence are influenced by various factors including preoperative tumor stage, histological type and type of surgical resection. Treated patients are at risk for developing a second lung primary, reported to be 1–4% per year, and therefore follow-up must be aimed at detecting not only recurrent cancer, but also a new, primary lung cancer. Different follow-up imaging strategies have been suggested, including conventional radiography, CT and/or PET scanning.
Keywords: Lung neoplasms, carcinoma, non-small cell lung, neoplasm recurrence, neoplasm metastasis
Introduction
Tumor recurrence is common following surgical resection for non-small cell lung cancer (NSCLC). Recurrences may occur locally, regionally and/or at distant sites. Locoregional recurrence is defined as a tumor within the operated hemithorax involving ipsilateral lymph nodes (including supraclavicular lymph nodes), the bronchial stump, and the pleura and chest wall [1]. Lung nodules along the surgical margin are considered locoregional metastases, whereas nodules remote from the surgical margin are usually considered distant metastases. The most common locations of distant metastases from recurrent lung cancer are similar to those seen at the time of initial presentation, i.e. brain, bone, liver and adrenals [2, 3].
If a localized recurrence is detected, the patient may be treated with repeat resection for attempted cure. Chemotherapy and/or radiation therapy may be used for palliation of symptoms or to increase life expectancy. The risks for recurrence are influenced by multiple, complex factors and are not fully understood [4]. Factors include tumor type, tumor stage and extent of surgical resection.
Knowledge and recognition of tumor recurrence patterns is important in the imaging follow-up of lung cancer. The purpose of this article is to review the patterns of recurrence of NSCLC as they relate to tumor histology and initial tumor stage, as well as to type of surgical resection. Benign post-therapy findings simulating tumor recurrence are also described, and the recommended imaging follow-up strategy is discussed.
Tumor recurrence in relation to histologic type
Among the various cell types observed in NSCLC, adenocarcinoma typically shows the highest rate of both distant and combined locoregional/distant recurrence [1, 2, 5]. Both squamous cell carcinomas and adenocarcinomas are somewhat more likely to recur at distant sites rather than locoregionally [5, 6] (Fig. 1).
Figure 1.
Liver metastasis 4 months following right pneumonectomy for poorly differentiated NSCLC. Chest CT (a) shows a fluid-filled pneumonectomy space (*), with no evidence of locoregional recurrence. CT of the upper abdomen (b) demonstrates a liver metastasis (arrow).
Although some reports state that locoregional recurrences are more commonly seen in squamous cell carcinomas compared to adenocarcinomas [5, 6], other studies have found contradictory results [7]. Other tumor types with high recurrence rates include large cell and epidermoid carcinomas [2, 5]. Interestingly, locoregional recurrences have not been reported in bronchioloalveolar cell carcinomas (BAC), excluding ipsilateral lung nodules [5]. Unfortunately, the tumor recurs with a high frequency in the lungs of patients who have undergone lung transplantation for this disease, and therefore transplantation in this setting is controversial [8].
Jang et al. reported recurrence rates at different sites for different tumor types. Pleural recurrence was seen in 9% and 7% of adenocarcinomas and large cell carcinomas, respectively, and only in 4% of squamous cell carcinomas. Mediastinal and hilar lymph node recurrence was seen at a higher rate in large cell cancers (21%) compared to squamous cell cancers (12%) and adenocarcinomas (8%), respectively. One published series reported that bronchial stump recurrences occurred exclusively in squamous cell carcinomas [5]. However, a different series found stump recurrences in 36% of adenocarcinomas, as well as 57% of squamous cell carcinomas [9] (Fig. 2).
Figure 2.
Bronchial stump recurrence following right upper and middle lobectomy for squamous cell lung carcinoma with negative surgical margins. Pre-operative CT (a) shows a right upper lobe mass. CT 8 months later (b), obtained to work up hemoptysis, demonstrates a soft tissue mass at the bronchial resection margin, extending into the trachea (arrow).
Tumor recurrence in relation to stage at time of resection
As expected, the rate of overall recurrence increases with higher initial T and N stages. Locoregional and/or distant tumor recurrence develops within 5 years after surgery in approximately 20–30% of patients with Stage I disease, 50% with Stage II disease, and 70–80% with Stage III disease [10]. Reported locoregional recurrence rates in the study of Jang et al. were 6% for Stage IA tumors, 16% for Stage IB, 36% for Stage IIA, 20% for Stage IIB, 36% for Stage IIIA and 33% for Stage IIIB cancers [5].
Not surprisingly, there is a significantly increased rate of recurrence in T2 tumors relative to T1 tumors and in tumors measuring larger then 5 cm compared to those measuring 3–5 cm [6]. It has been reported that the rate of chest wall recurrence increases with higher T stage (Fig. 3), and recurrence within pleura and hilar and mediastinal lymph nodes is related to higher N stage [5]. The locoregional recurrence rate in patients under going sleeve lobectomy is reportedly influenced by N status, with 14% recurrence in N0 disease compared to 23% in N1 and 42% in N2 disease [11].
Figure 3.
Chest wall recurrence 3 months following left upper lobectomy and chest wall resection for Stage IIB (T3N0M0) NSCLC with negative surgical margins.
In general, post-operative tumor spread to distant sites is more common than locoregional recurrence for patients with Stage I, II and III disease. For example, it is reported that Stage I tumors show distant tumor spread in 20% of cases, compared to a rate of 7% for locoregional recurrence. For Stage II lesions, the corresponding figures are 49% for distant spread compared to 32% for locoregional disease [2, 6]. Combined distant, local and regional recurrence, within an individual patient, has been reported in up to 19.7% of patients [2]. The disease-free interval is reported to be similar for patients with either local or distant recurrence [6].
Tumor recurrence in relation to type of therapy
Patterns and frequency of recurrence may vary according to the type and extent of surgery or other type of therapy. Tumors treated with radiation therapy may recur outside of the radiation port (Fig. 4). Limited surgery, for example using wedge resection or segmentectomy without mediastinal lymph node dissection, typically shows an increased rate of tumor recurrence, with frequencies ranging up to 50% [6, 12]. Lymphatic invasion adjacent to the primary tumor has been reported in 25% of Stage I tumors measuring less than 1 cm and in 57% of Stage I tumors measuring more than 3 cm. However, when there were hilar lymph node metastases (N1 disease), local lymphatic invasion was as high as 85% for tumors measuring up to 3 cm in one study [13]. Therefore, a limited resection may leave tumor cells behind; residual tumor cells may grow locally, at the staple line (Fig. 5) [13], spread to regional lymph nodes, or metastasize to distant sites. Furthermore, the tumor may remain behind in unresected regional lymph nodes, if a lymph node dissection has not been performed, leading to local nodal tumor recurrence.
Figure 4.
Tumor recurrence outside radiation port. CT shows left paramediastinal radiation fibrosis (arrow) (a) after left upper lobectomy and radiation therapy for squamous cell carcinoma. Also seen are a right upper lobe mass (white solid arrow) and right hilar lymph node enlargement (white dotted arrow), representing recurrent tumor (b).
Figure 5.
Tumor recurrence (arrow) at staple line 2 years after wedge resection for NSCLC.
A positive surgical margin occurring at either partial or complete lung resection may result in tumor recurrence at this margin, particularly if no post-operative radiation therapy is given. This may involve lung, bronchial stump, pleura and/or chest wall.
Contralateral shift of the tracheal air column suggests recurrence on a conventional radiograph [14]. However, CT is more sensitive for evidence of recurrence within mediastinal lymph nodes or the pneumonectomy space.
Benign imaging findings after treatment
It is important to be aware of normal, benign findings that occur after surgery and/or radiation therapy, and to distinguish such findings from those indicative of recurrent tumor. Following pneumonectomy, the ipsilateral hemithorax normally fills with organized fluid, appearing as low attenuation material on CT (Figs 1, 3 and 6); the presence of a mass within the low attenuation material is suggestive of local tumor recurrence [14, 15] (Fig. 3). Post-radiation pulmonary necrosis is an uncommon, severe complication of adjuvant post-operative radiation therapy. It manifests as cavitation within a fibrotic space (usually in the lung apex) and occurs 1–7 years following treatment. Such an appearance may simulate recurrent tumor [16]; the diagnosis is generally one of exclusion, usually made after a negative biopsy and/or stability on follow-up imaging (Fig. 7).
Figure 6.
Tracheal recurrence 6 months after pneumonectomy for squamous cell cancer. CT demonstrates a soft tissue mass in the distal trachea (arrow). Note the normal, fluid-filled pneumonectomy space (*).
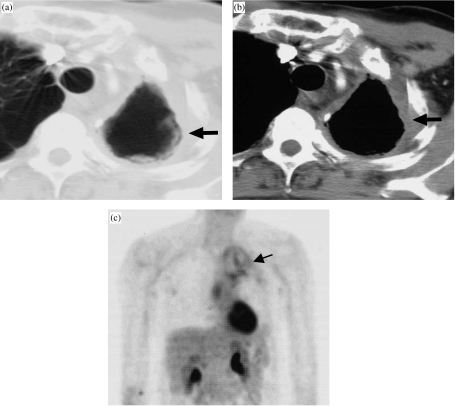
Figure 7.
Benign, post-radiation necrosis simulating recurrent, cavitary neoplasm. CT (a, b) obtained 18 months after left upper lobectomy and post-operative radiation therapy for a large cell neuroendocrine carcinoma shows a left apical cavity surrounded by a rind of soft tissue (arrow). A projection PET scan reveals low level uptake within the rind (arrow).
Imaging follow-up
The main purpose of imaging follow-up after treatment is to detect recurrent tumor. Survival following locoregional or distant recurrence is poor and is therefore generally treated with radiation and/or chemotherapy, rather than with surgery [17]. Sometimes, however, no treatment is given until the patient becomes symptomatic.
In addition to the high risk of developing recurrent cancer, treated patients have a 1–4% risk per year for developing a second primary bronchogenic carcinoma [18]. Follow-up imaging may detect such a new (metachronous) primary lung cancer, allowing early, definitive therapy. If surgery is performed, the extent of resection is generally dependent upon the degree of pulmonary reserve.
The recommendation for follow-up imaging after treatment is based on the high incidence of recurrence during the first 2 years following therapy. However, there is no consensus regarding the routine use of CT in this setting; according to most guidelines, chest CT should be omitted, because many recurrences are extrathoracic and patients tend to have a poor outcome, either with or without additional therapy [17]. It has been suggested that follow-up imaging be performed using chest radiography, reserving chest CT for patients with symptoms or an abnormal chest radiograph [19]. However, some investigators have suggested performing a baseline, post-operative CT for future reference, whereas others obtain routine, annual surveillance chest CT scans [17]. At some institutions, chest radiographs are performed every 4 months for 2 years, then every 6 months thereafter, in addition to annual CT, to allow early detection of a new, early stage, primary lung cancer [18].
FDG-PET imaging has a role in distinguishing persistent or recurrent tumor from post-treatment scarring or fibrosis. It is more sensitive than chest CT and conventional radiography in detecting recurrent tumor (sensitivity of 97–100%) [20–22] (Fig. 8). However, it has a specificity of 62–100%, sometimes yielding false-positive results from active inflammation, particularly in the acute post-operative or post-radiation stage. Therefore, FDG-PET scanning should be obtained no earlier than 4–5 months following radiation therapy [20].
Figure 8.
Recurrent cancer at bronchial margin detected with PET scanning. PET scan (a) 8 months after right lower lobectomy for squamous cell cancer shows abnormal radiotracer uptake in the right hilum (arrow), corresponding to minimal and equivocally abnormal soft tissue at the bronchial margin on CT (arrow) (b). Follow-up CT 7 months later (c) demonstrates interval enlargement of recurrent tumor mass (arrow).
Conclusions
The pattern and frequency of lung cancer recurrence following surgery is related to tumor staging and histologic type, as well as to the type of surgical procedure preformed. Familiarity with the different patterns of local recurrence within the chest, as well as the appearance of normal treated chest, is essential in following patients with treated lung cancer. However, the usefulness of routine chest CT scans for surveillance after surgery is debated; according to most published guidelines, surveillance with chest radiography is sufficient in the asymptomatic patient. On the other hand, others advocate annual follow-up CT.
Key points
The risk for recurrent lung cancer is directly related to the tumor T and N stage at the time of resection, as well as to the histologic type and the extent of resection.
Post-surgical findings and radiation effects may simulate tumor recurrence on imaging; therefore, familiarity with the post-treatment appearance is important.
Treated patients have a life long risk of developing a second primary lung cancer, and follow-up should be aimed at detection of a new primary neoplasm, as well as recurrence of the original tumor.
References
- 1.Yano T, Yokoyama H, Inoue T, et al. The first site of recurrence after complete resection in non-small-cell carcinoma of the lung. Comparison between pN0 disease and pN2 disease. J Thorac Cardiovasc Surg. 1994;108(4):680–3. [PubMed] [Google Scholar]
- 2.Cangemi V, Volpino P, D’Andrea N, et al. Local and/or distant recurrences in T1-2/N0-1 non-small cell lung cancer. Eur J Cardiothorac Surg. 1995;9(9):473–8. doi: 10.1016/s1010-7940(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 3.Quint LE, Tummala S, Brisson LJ, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg. 1996;62(1):246–50. doi: 10.1016/0003-4975(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 4.Spiro SG, Porter JC. Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med. 2002;166(9):1166–96. doi: 10.1164/rccm.200202-070SO. [DOI] [PubMed] [Google Scholar]
- 5.Jang KM, Lee KS, Shim YM, et al. The rates and CT patterns of locoregional recurrence after resection surgery of lung cancer: correlation with histopathology and tumor staging. J Thorac Imaging. 2003;18(4):225–30. doi: 10.1097/00005382-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120–9. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 7.Baldini EH, DeCamp MM Jr, Katz MS, et al. Patterns of recurrence and outcome for patients with clinical stage II non-small-cell lung cancer. Am J Clin Oncol. 1999;22(1):8–14. doi: 10.1097/00000421-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Zorn GL Jr, McGiffin DC, Young KR Jr, Alexander CB, Weill D, Kirklin JK. Pulmonary transplantation for advanced bronchioloalveolar carcinoma. J Thorac Cardiovasc Surg. 2003;125(1):45–8. doi: 10.1067/mtc.2003.72. [DOI] [PubMed] [Google Scholar]
- 9.Miura H, Konaka C, Kato H, Kawate N, Taira O. Recurrence at the bronchial stump after resection of lung cancer. Ann Surg. 1994;219(3):306–9. doi: 10.1097/00000658-199403000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downey RJ. Follow-up of patients with completely resected lung cancer. Chest. 1999;115(6):1487–8. doi: 10.1378/chest.115.6.1487. [DOI] [PubMed] [Google Scholar]
- 11.Tronc F, Gregoire J, Rouleau J, Deslauriers J. Long-term results of sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg. 2000;17(5):550–6. doi: 10.1016/s1010-7940(00)00405-x. [DOI] [PubMed] [Google Scholar]
- 12.Kodama K, Doi O, Higashiyama M, Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg. 1997;114(3):347–53. doi: 10.1016/S0022-5223(97)70179-X. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose Y, Yano T, Yokoyama H, Inoue T, Asoh H, Katsuda Y. The correlation between tumor size and lymphatic vessel invasion in resected peripheral stage I non-small-cell lung cancer. A potential risk of limited resection. J Thorac Cardiovasc Surg. 1994;108(4):684–6. [PubMed] [Google Scholar]
- 14.Glazer HS, Aronberg DJ, Sagel SS, Emami B. Utility of CT in detecting postpneumonectomy carcinoma recurrence. Am J Roentgenol. 1984;142(3):487–94. doi: 10.2214/ajr.142.3.487. [DOI] [PubMed] [Google Scholar]
- 15.Peters JC, Desai KK. CT demonstration of postpneumonectomy tumor recurrence. Am J Roentgenol. 1983;141(2):259–62. doi: 10.2214/ajr.141.2.259. [DOI] [PubMed] [Google Scholar]
- 16.Mesurolle B, Qanadli SD, Merad M, et al. Unusual radiologic findings in the thorax after radiation therapy. Radiographics. 2000;20(1):67–81. doi: 10.1148/radiographics.20.1.g00ja1167. [DOI] [PubMed] [Google Scholar]
- 17.Colice GL, Rubins J, Unger M. American College of Chest Physicians. Follow-up and surveillance of the lung cancer patient following curative-intent therapy. Chest. 2003;123(Suppl. 1):272S–83S. doi: 10.1378/chest.123.1_suppl.272s. [DOI] [PubMed] [Google Scholar]
- 18.Lamont JP, Kakuda JT, Smith D, Wagman LD, Grannis FW Jr. Systematic postoperative radiologic follow-up in patients with non-small cell lung cancer for detecting second primary lung cancer in stage IA. Arch Surg. 2002;137(8):935–8. doi: 10.1001/archsurg.137.8.935. [DOI] [PubMed] [Google Scholar]
- 19.Younes RN, Gross JL, Deheinzelin D. Follow-up in lung cancer: how often and for what purpose? Chest. 1999;115(6):1494–9. doi: 10.1378/chest.115.6.1494. [DOI] [PubMed] [Google Scholar]
- 20.Erasmus JJ, McAdams HP, Patz EF Jr. Non-small cell lung cancer: FDG-PET imaging. J Thorac Imaging. 1999;14(4):247–56. doi: 10.1097/00005382-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bury T, Corhay JL, Duysinx B, et al. Value of FDG-PET in detecting residual or recurrent nonsmall cell lung cancer. Eur Respir J. 1999;14(6):1376–80. doi: 10.1183/09031936.99.14613769. [DOI] [PubMed] [Google Scholar]
- 22.Hicks RJ, Kalff V, MacManus MP, et al. The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42(11):1605–13. [PubMed] [Google Scholar]