Abstract
Intensity-modulated radiotherapy (IMRT) is one of the most important recent developments in oncology. It enables precise conformation of the radiation dose to the target volume. It has the potential to significantly reduce long-term morbidity and improve local control. This article explains the basic principles of IMRT in comparison to other planning techniques. The current clinical data are presented and future lines of research are discussed.
Keywords: Intensity-modulated radiotherapy, radiotherapy planning
Introduction
Intensity-modulated radiotherapy (IMRT) is one of the most important advances in oncology of the past decade. Improvements in both computer technology and imaging techniques have enabled the rapid development of this exciting treatment modality. There is now extensive research ongoing world-wide to investigate the applications of this new technique.
An ideal radiotherapy treatment delivers a high dose of radiation to the tumour but minimal dose to the surrounding normal tissue. For many tumours there is a clear relationship between radiation dose and the probability of tumour control, but the tumour dose is often limited by the radiation tolerance of surrounding structures. By conforming more precisely to the selected target IMRT may allow more normal tissue to be spared than with other techniques. This provides the possibility of both reducing late toxicity and increasing the delivered dose which could lead to improved tumour control and survival.
This review will describe the current status of IMRT, highlight the recent advances in imaging that are incorporated into the planning process and emphasise the areas of future research.
Three-dimensional planning techniques
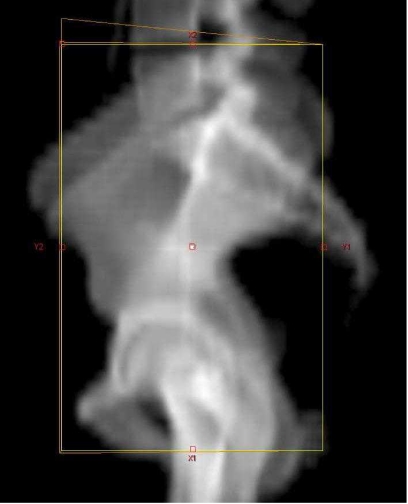
The first step in planning a radiotherapy treatment is the accurate definition of the target volume. The different radiotherapy techniques available are demonstrated using the example of a patient with prostate cancer who is to have both pelvic lymph nodes and the prostate gland irradiated. Conventional radiotherapy defines the area to be treated in relation to bony landmarks as shown in Fig. 1. Multiple overlapping beams are then used to create a brick-shaped central region of high dose distribution. This method is simple and quick but results in the irradiation of significant volumes of the small bowel, rectum and bladder.
Figure 1.
Conventional field (yellow) defined by bony landmarks.
Conformal radiotherapy
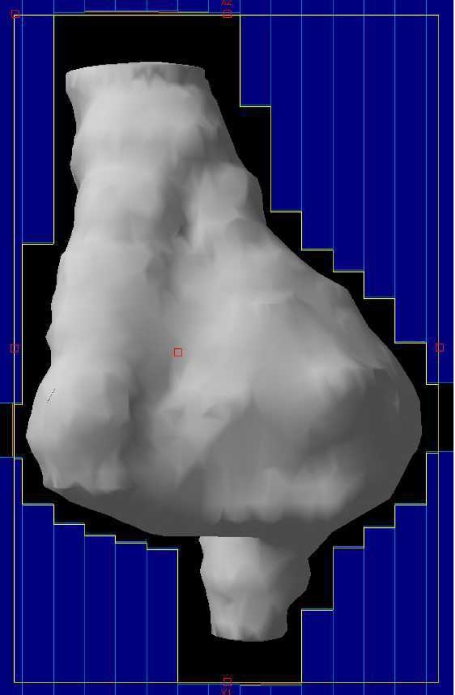
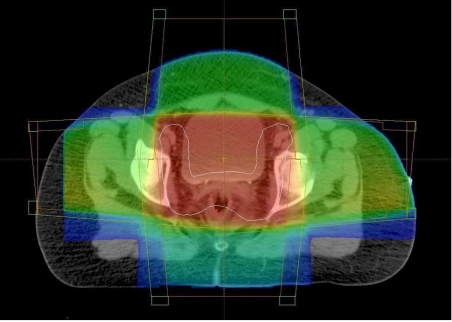
The introduction of CT scanning dramatically improved the accuracy of defining both the tumour and organs at risk. Tumour is identified and drawn onto each cross-sectional image creating a reconstructed three-dimensional target volume (Fig. 2). The radiation beams are then shaped to fit this volume and to provide shielding to the normal tissues. This technique allows an improved spatial distribution of the dose, but is still unable to exclude normal tissues surrounded by tumour (Fig. 3).
Figure 2.
Conformal field. The volume is outlined on each CT slice and a three-dimensional volume is created (white). Fields are shaped with MLC leaves (blue).
Figure 3.
Conformal dose colour-wash. The target volume is contoured in white. The high dose region (red) is brick-shaped and includes part of the bladder.
Conformal radiotherapy is now in routine use for the treatment of many tumour sites including head and neck, brain, lung, prostate and bladder cancer. The predicted reduction in toxicity due to the improved conformity has been confirmed in clinical studies. Dearnaley et al. randomised patients with prostate cancer between conformal and conventional radiotherapy. Long-term rectal toxicity was reduced from 15% to only 5% in the conformal arm [1]. This reduction in morbidity has enabled the use of escalating dose to treat prostate cancer. Investigators at Memorial Sloane Kettering Hospital increased the prescribed dose from 64 to 81 Gy resulting in improved tumour control but an increased risk of toxicity. To treat with these high doses, it is necessary to further reduce the volume of rectum irradiated. This would only be possible with the introduction of IMRT.
Intensity-modulated radiotherapy
IMRT is an advanced form of three-dimensional conformal radiotherapy. It is of particular value for target volumes with concave or complex shapes with close proximity to radiosensitive normal structures [2]. It has two key additional features compared to conformal radiotherapy:
Non-uniform intensity of the radiation beams.
Computerised inverse planning.
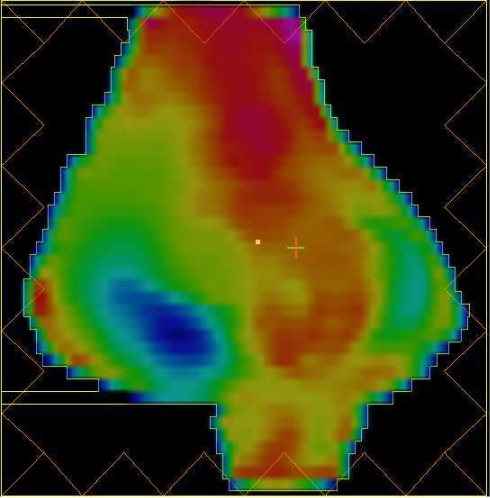
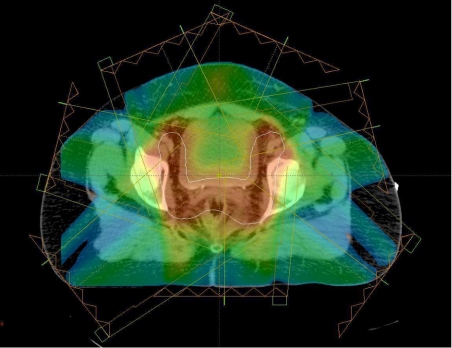
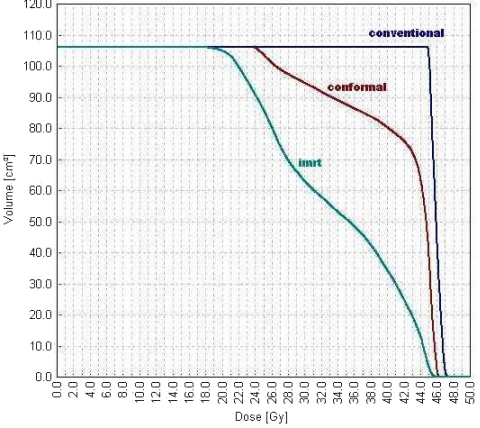
Variable radiation intensity is generated across each beam, in contrast to the uniform intensity used in other radiotherapy techniques. Each beam is subdivided into hundreds of beamlets, each with an individual intensity level, enabling a very complex pattern to be constructed (Fig. 4). The use of several beams can build up a highly conformal dose distribution, allowing precise shaping to a curved target and thus further sparing of the normal tissues. In Fig. 5, the high dose region fits the nodal target volume precisely, protecting the centrally situated bladder and small bowel. The dosimetric benefit is assessed using a comparative dose-volume histogram that shows the volume of bladder irradiated when the patient is treated with conventional, conformal and IMRT plans (Fig. 6). The volume receiving more than 40 Gy is reduced by 20% using conformal radiotherapy and there is a further 45% reduction using IMRT, demonstrating the dramatic improvement in dosimetry that can be achieved with this technique.
Figure 4.
IMRT fluence represented with colour. The field is divided into multiple beamlets with variable intensities.
Figure 5.
IMRT dose colour-wash. The high dose region (red) conforms to the target volume (white) in a concave shape reducing the bladder and bowel dose.
Figure 6.
Comparative dose-volume histogram for the three techniques.
Advantages of IMRT
Improved target conformity, particularly for concave target volumes
Can produce intentional dose inhomogeneity—dose-painting
Increases normal tissue sparing
Enables dose escalation
Can compensate for missing tissue.
Disadvantages of IMRT
Increased clinician time for target and organ outlining
Needs extensive quality assurance programme
Increased machine treatment time
Increased planning time (initially)
Increased total body irradiation dose.
IMRT planning
Inverse planning
One of the key features that distinguishes IMRT from other radiotherapy techniques is the use of computerised inverse planning. Conformal radiotherapy is forward planned and reliant on the skills of the treatment planner to decide the number, shape and orientation of the beams. Inverse planning, in contrast, specifies the plan outcome in terms of the tumour dose and normal structure dose limits. The computer system then adjusts the beam intensities to find a configuration best matched to the desired plan.
Each beamlet is traced through the patient producing an initial dose distribution. A small change is then made in the weighting of a single beamlet and this alteration is accepted if it results in an improved distribution. This process is repeated for all beamlets during a single cycle (iteration) and should result in an improved plan. The iterative process is repeated for many cycles until no further improvement is seen. This results in the optimal intensity across each beam to produce the specified dose distribution. The calculations in this process would be impossible with a forward planned manual technique.
Currently, this planning process may be lengthy because the adjustments required to produce acceptable plans are not intuitive. With experience, total planning times are likely to be significantly reduced as centres develop standard field arrangements for use in particular tumour sites.
Dose prescription—simultaneous modulated accelerated RT
The clinician must specify the required dose to be delivered to the target volume and also define the dose limits for the organs at risk. With conventional and conformal treatments, tumours in close proximity to radiosensitive structures often require very complex treatment plans. The dose to the tumour may need to be reduced to prevent unacceptable late complications. Frequently, it is necessary to divide the treatment course into several phases, each of which has different field arrangements. IMRT allows the allocation of different dose targets for treatment (‘dose-painting’) creating an intentionally inhomogeneous dose distribution. It is possible to simultaneously boost the areas of higher risk allowing the patient to be treated in a single phase. This technique of simultaneous modulated accelerated RT (SMART) is very efficient to plan and deliver, can escalate the tumour dose and will decrease total treatment time.
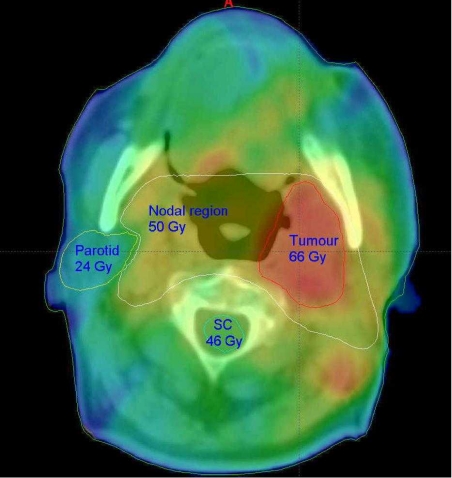
The process of dose-painting is illustrated in Fig. 7 for a patient with an oropharyngeal tumour. The primary tumour is prescribed a dose of 66 Gy and the cervical nodes which are a potential site of microscopic involvement are to receive the lower dose of 50 Gy. Spinal cord and brain stem which are adjacent to the target volume are limited to a maximum dose of 46 Gy. Salivary glands are very radiosensitive, and at doses in excess of 25–30 Gy permanent xerostomia may occur which is often distressing for the patient. In oropharyngeal tumours conventional and conformal radiotherapy would inevitably encompass both parotid glands. IMRT, however, can deliver the required dose in a single phase whilst also protecting the contralateral parotid gland and reducing the risk of long-term morbidity.
Figure 7.
Dose-painting for an oropharyngeal tumour. The target doses for the spinal cord (sc), parotid, lymph nodes and tumour are marked. The IMRT plan delivers a high dose (red) to the tumour and low dose (blue) to the contralateral parotid gland.
IMRT treatment delivery
A number of diverse techniques have been developed to deliver IMRT. The two most common methods of segmental IMRT and dynamic IMRT use multi-leaf collimators (MLC). The first MLC system was developed in 1948 for delivery of dynamic conformal treatment and has become standard on modern linear accelerators. It consists of movable tungsten leaves that can block part of the radiation field. Typically leaves are 5–10 mm wide arranged in opposing pairs that can be positioned under computer control to create an irregular field that conforms to the tumour shape, and shields normal tissues. It is used for conformal radiotherapy (Fig. 2), and is an essential element of linear accelerator delivered IMRT.
Segmental MLC
This method has evolved directly from conformal radiotherapy. For each beam orientation, several different MLC-shaped fields (segments) are created. A modulated field intensity is achieved by summing all the segments. The radiation is only turned on when the segments are in position and thus this method is known as step-and-shoot. This is now available from most linear accelerator manufacturers and is likely to become the most widely implemented technique.
Dynamic MLC
In contrast, with dynamic MLC-IMRT the MLC leaves are in continuous motion during treatment of each field. At a fixed beam angle, each pair of opposing MLC leaves is swept across the target volume under computer control to produce the desired fluence profile. Variation of the speed and distance between leaves delivers the desired intensity of radiation to the specified point. This can produce more conformal dose distributions than segmental IMRT and is therefore more desirable for complex problems. This method does continue to deliver radiation while the MLC is in the beam, producing more leakage and increasing the total body dose.
Tomotherapy
IMRT can be delivered with a fan beam, in a similar technique to CT scanning. The beam is collimated to a narrow slit and then modified with moving leaves as the gantry rotates around the patient. A helical tomographic system has also recently been developed, similar to helical CT scanners. This method produces highly complex shapes and has been used for the majority of the IMRT treatments world-wide. However, implementation in a new department would require additional equipment, and thus the MLC based techniques are likely to become predominant.
Imaging and IMRT
Tumour localisation
Accurate identification of the tumour is important for all radiotherapy techniques, but becomes even more essential with IMRT as the irradiated volume becomes so closely aligned to the target volume. CT scanning is the standard imaging modality for radiotherapy planning. It is widely available, geometrically accurate and provides electron density information that is necessary for planning algorithms. The main limitation of CT is that it cannot always identify the tumour adequately and other imaging modalities are needed to provide additional information. MRI is increasingly used for staging of pelvic, head and neck and brain tumours, due to the excellent soft tissue contrast provided. At present, however, MRI cannot be used directly for radiotherapy planning due to geometric distortion and lack of electron density information.
Functional imaging techniques such as PET, SPECT and MRS are also potential tools for treatment planning, providing valuable information about patient and tumour physiology rather than anatomy. PET can be used with different tracers to assess microscopic nodal involvement and to define hypoxic or significantly proliferative areas within the tumour that could require boosting. However, these modalities have poor spatial resolution and need to be combined with CT imaging for radiotherapy planning.
Image fusion
Image registration and fusion are techniques used to aid tumour localisation. Different imaging modalities are superimposed using internal landmarks or by matching on a voxel to voxel basis [3]. This is used most frequently for head and neck and brain tumours. Although clearly visible on MRI, the full extent of the tumour is usually not seen on the CT scan. The tumour contour drawn on the MRI is automatically transferred onto the corresponding CT image. This method allows the use of all relevant modalities to define the tumour, and the CT images can still be used for accurate planning calculations.
Tumour and patient movement
The planned target volume must ensure adequate cover of all disease throughout the course of treatment and therefore must take into account all potential movements of both the tumour and patient. Conventionally, a margin is added to the radiologically defined volume of macroscopic disease and areas considered at risk of microscopic spread. The margin must allow for both the daily variation in patient set-up and internal organ motion. An inappropriate margin will lead to underdosing of the tumour and/or overdosing of the surrounding organs at risk. As IMRT tightly conforms the high dose region to the target volume whilst minimising the dose to normal structures, there is usually a more rapid fall-off of dose in all directions outside the target volume when compared to conformal radiotherapy. Patient or tumour motion is therefore of particular importance in IMRT and extra care is needed to limit patient movement with reproducible patient immobilisation.
Image guidance
Motion of the tumour between daily fractions is significant for many tumour sites and could result in a geographical miss. Many studies on prostate cancer have shown that the prostate can move up to 2 cm between treatments and the position is dependent on rectal filling [4]. Image guidance can improve geometrical accuracy by adjusting the fields to the daily position of the target. Radiographic imaging of implanted radio-opaque markers is now feasible with electronic portal imaging. Daily ultrasound imaging can also localise the prostate immediately before treatment [5].
A promising approach is the development of the cone-beam CT linear accelerator. A kilovoltage imaging system is integrated into the linear accelerator. A tomographic reconstruction of the volume is performed before treatment, and correction can be made for both target position and the set-up error. At present, routine use is not practicable due to the prolonged machine time required, but several centres are using this for research purposes [6].
Clinical experience of IMRT
IMRT is a new but rapidly developing field of research. There are many published reports of comparative planning studies that demonstrate the superior dose distribution that can be achieved using IMRT for various tumour sites, including prostate, breast, glioma, medulloblastoma, gynaecological and head and neck tumours. However, so far there are very few clinical studies that have demonstrated the predicted clinical benefit and to date there are no reported prospective randomised clinical trials.
Head and neck cancer
Radiotherapy treatment for head and neck cancers is frequently associated with long-term morbidity. These tumours have great potential to benefit from IMRT because of the complexity and concavity of the target volume and the proximity of radiosensitive dose-limiting structures. Several recent studies have demonstrated improved target doses and sparing of the normal tissues. Parotid-sparing IMRT, as shown in Fig. 7, was first used at the University of Michigan. The spared parotid glands recovered to 63% of their pre-treatment level, compared to only a 3% recovery rate in glands treated with conventional doses. A review of 126 patients treated with IMRT showed good local control, with no increase in relapse in the region adjacent to the spared parotid glands [7].
Prostate cancer
A dose response relationship has been shown in prostate cancer. Dose escalation was enabled by the introduction of conformal radiotherapy, and several groups have used IMRT to further increase the dose. A low rate of acute and late toxicity is reported, but the data are still too immature for survival assessments. In addition to dose escalation, pelvic nodal irradiation with a boost to the primary tumour is now possible as a single-phase treatment using IMRT.
Breast cancer
Post-operative radiotherapy for breast cancer gives significant benefits in local control and survival, but long-term follow up studies have shown increased side effects including cardiac disease, lung damage, breast distortion, rib fractures and pain. IMRT can improve the dose uniformity by compensating for missing tissue and consequently improves the cosmetic outcome. It is possible to boost the tumour bed using IMRT, and this issue will be examined in a UK randomised trial of partial breast irradiation.
Discussion
IMRT represents one of the most important advances in radiotherapy in recent years. It offers the potential to improve clinical outcome and reduce morbidity. It is still developing as a treatment modality but once established it is likely to reduce planning and treatment times. Tumour localisation using optimal imaging, management of organ motion and image guidance on a daily basis are promising areas of research which will need to be incorporated into future IMRT treatments.
Implementation of IMRT requires extensive co-operation between the multi-disciplinary team. A comprehensive quality assurance programme needs to be developed and there must be continuous evaluation of procedures. There is currently a lack of long-term clinical data, and the effect of increasing the total body radiation dose is uncertain. Large scale randomised clinical trials are needed to confirm the predicted benefits. This is a rapidly evolving field and much work still remains to determine the best use of these exciting technical and planning capabilities.
References
- 1.Dearnaley DP, Khoo VS, Norman AR, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353(9149):267–72. doi: 10.1016/S0140-6736(98)05180-0. [DOI] [PubMed] [Google Scholar]
- 2.IMRT Collaborative Working Group Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51(4):880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 3.Emami B, Sethi A, Petruzzelli GJ. Influence of MRI on target volume delineation and IMRT planning in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2003;57(2):481–8. doi: 10.1016/s0360-3016(03)00570-4. [DOI] [PubMed] [Google Scholar]
- 4.Van Herk M, Bruce A, Kroes AP, Shouman T, Touw A, Lebesque JV. Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int J Radiat Oncol Biol Phys. 1995;33(5):1311–20. doi: 10.1016/0360-3016(95)00116-6. [DOI] [PubMed] [Google Scholar]
- 5.Lattanzi J, McNeeley S, Pinover W, et al. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int J Radiat Oncol Biol Phys. 1999;43(4):719–25. doi: 10.1016/s0360-3016(98)00496-9. [DOI] [PubMed] [Google Scholar]
- 6.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53(5):1337–49. doi: 10.1016/s0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- 7.Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55(2):312–21. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]