Abstract
The role of endoscopic ultrasound (EUS) in the detection of pancreatic islet cell tumours is reviewed. Functioning islet cell tumours are frequently small at presentation (90%<2 cm). Advances in cross-sectional imaging with CT and MRI have resulted in improved detection rates of these small lesions. The sensitivity of EUS in the detection of insulinoma is similar to helical or multislice CT, i.e. between 82 and 94%, while a combination of both techniques is reported to identify 100% of tumours. EUS may be considered a primary diagnostic tool in these patients. EUS has a secondary role in the detection of gastrinomas as over 50% are malignant and 5% extra-pancreatic in position. CT should be used as a first-line investigation. EUS is valuable in problem solving in these patients. EUS has a role in staging large tumours prior to surgery. EUS-guided fine needle aspiration may provide cytological confirmation of the nature of a tumour prior to surgery.
Keywords: Insulinoma, gastrinoma
Introduction
Islet cell tumours are uncommon neoplasms occurring in approximately 1–5 cases/million population per year [1]. They are classified as functioning (85%) and non-functioning tumours (15%).
Non-functioning tumours frequently present at an advanced stage—either as a large pancreatic mass when benign, or with metastatic disease to the liver or lymph nodes when malignant. However, with the increasing use of cross-sectional imaging, they are not infrequently discovered as an incidental finding when still relatively small. The role of imaging in these tumours is to identify characteristic features that may enable differentiation from pancreatic adenocarcinoma and to detect signs of malignancy.
Functioning tumours are named after the hormone they secrete. The most common tumours are insulinomas (60%) and gastrinomas (18%) with other tumours—somatostatinomas, vipomas and glucagonomas—each representing only approximately 1% of the total. The malignant potential of these tumours varies with the cell type from 10 to 90%. Functioning tumours are typically small at presentation and the diagnosis is established by the associated typical biochemical and clinical abnormalities. The challenge to the radiologist is the localisation of these tiny lesions.
The requirement for accurate pre-operative localisation has not been universally accepted. Historically, non-invasive imaging techniques have been relatively insensitive and some experts believe that in the context of an established biochemical diagnosis, most functioning tumours can be identified at surgery by pancreatic palpation and inspection, often accompanied by intra-operative ultrasound. Others believe that the precise pre-operative localisation of functioning tumours, the detection of multiple lesions and the identification of signs of malignancy allow for more accurate surgical planning and pre operative patient counselling.
There is considerable debate as to the optimal imaging strategy to be adopted in these patients. The search for small lesions has frequently involved a multiplicity of diagnostic tests including percutaneous ultrasound, CT, MRI, somatostatin receptor scanning, angiography and venous sampling [2]. Technical advances, particularly in MRI and CT, have resulted in a significant improvement in image quality and although early studies reported disappointing sensitivities, recent studies have reported improved detection [3].
The potential role of endoscopic ultrasound (EUS) in the search for small islet cell tumours was highlighted in 1992 by Rösch et al. [4] who excluded from their study any patient in whom transabdominal ultrasound or CT was positive, thereby eliminating large tumours and focusing on the real problem of tumours less than 2 cm in diameter. Using EUS they correctly identified 32 of 39 surgically proven intra-pancreatic tumours (sensitivity 82%) with a specificity of 95%. These impressive results led to the recommendation that once the clinical diagnosis has been established, EUS should be used at an early stage in the localisation of neuroendocrine tumours of potential pancreatic origin.
Technique: EUS
Most reported EUS studies employ the radial sector scanner (GF-UM20, Olympus, Tokyo, Japan). A side-viewing endoscope is combined with a 7.5 and 12 mHz ultrasound transducer located at the end of the scope distal to the side viewing optics. The field of view is 360∘ and is orthogonal to the long axis of the scope.
Linear scanning endoscopes (Pentax FG 32/38) are also available which employ a 5–7.5 mHz transducer with a 105∘ section of scanning. The field of view with these instruments is more restricted and anatomic localisation can be more difficult. However, the major benefit of these scopes is that the scanning plane is parallel to the shaft of the instrument and therefore facilitates the accurate placement of a needle for diagnostic purposes.
Examinations are undertaken with patient sedation and the patient in the left lateral decubitus position. The endoscope is introduced initially into the descending duodenum and ultrasound examination is performed by withdrawing the instrument, visualising in turn the uncinate process and pancreatic head through the duodenal wall. The body and tail of the pancreas are investigated with the endoscope withdrawn into the stomach. A fluid interface is provided by means of a water-filled balloon around the transducer and also, in selected patients, by filling the stomach with water. The length of the procedure can vary between 10 and 40 min.
Insulinoma
Insulinomas are the most common type of functioning islet cell tumour. Over 90% of these lesions are benign and intra-pancreatic in position, with an equal distribution in the head, body and tail. The majority are solitary and 60–75% of tumours are less than 1.5 cm in diameter. Forty percent of lesions are less than 1 cm and can be as small as 2 mm, and are difficult to detect. Five to ten percent of lesions are multiple and in 5–10% of cases, hypoglycaemia may be due to nesidioblastosis or islet cell hyperplasia which is characterised by a proliferation of abnormal B cells throughout the pancreas.
Two to five percent of tumours may be ectopic in position and can be difficult to localise. A small proportion are pedunculated lesions. Malignant lesions tend to be larger and the majority of malignant islet cell tumours are greater than 3 cm in diameter at presentation [1, 2].
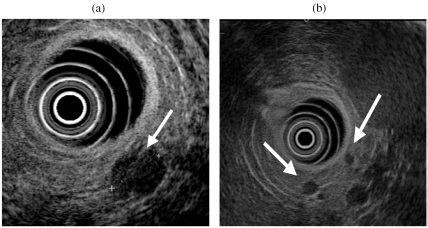
The typical appearance of an of insulinoma on EUS is of a small uniformly hypoechoic mass with a clearly defined distinct margin which is easily identified against the normal pancreatic parenchyma [5–7] (Fig. 1). The reported sensitivity of EUS ranges between 82 and 94% [4–9].
Figure 1.
(a) Typical EUS appearance of insulinoma: a well defined hypoechoic 1.5 mm mass in the mid body of the pancreas. (b) Two very small insulinomas (arrowed) measuring less than 5 mm in the distal pancreas.
Several studies report a lower sensitivity for detection of lesions in the pancreatic tail where the sensitivity is approximately 50–60% as compared with 95 and 98% for tumours of the head and body of the pancreas, respectively. This may reflect the anatomic disadvantage of scanning the tail from the stomach in patients who have increased retroperitoneal fat [4, 10].
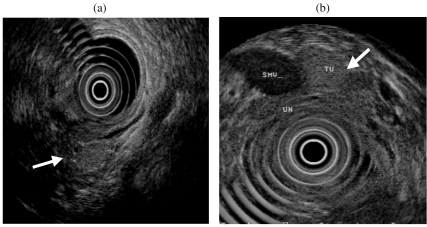
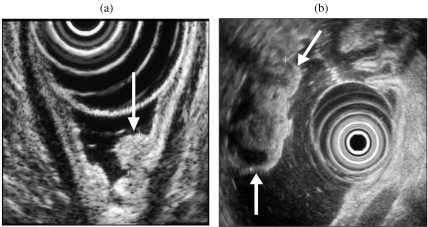
Because approximately 95% of insulinomas are pancreatic in location, a negative EUS examination is less reliable in excluding a pancreatic tumour (negative predictive value 43%) [9] and reflects the fact that isoechoic tumours and those which are pedunculated may be more difficult to detect (Fig. 2).
Figure 2.
(a) Isoechoic insulinoma in the pancreatic head (arrowed). (b) Pedunculated insulinoma (Tu) arising from the uncinate process (UN). SMV, superior mesenteric vein.
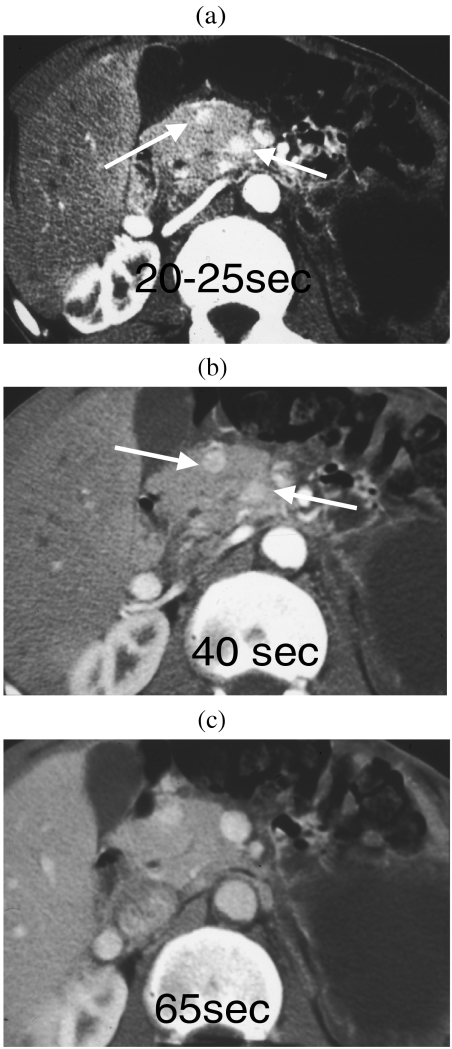
The sensitivity of CT in the detection of islet cell tumours is highly dependent on the techniques employed. Studies using contrast-enhanced helical CT report sensitivities between 65 and 82% [11–13]. Two recent studies employing multidetector, multiphase contrast-enhanced thin section CT have reported sensitivities of 83% [14] and 94% [7, 14] in the detection of insulinomas. Insulinomas are typically hyperattenuating on at least one phase of contrast enhancement—typically on the late arterial (25 s) or pancreatic phase (35–40 s) of imaging but occasionally in the portal venous phase [14]. In the analysis of the false-negative examinations (6/30), two tumours were revealed as isodense and pedunculated, three were in close proximity to vessels and one had a cystic appearance. The combination of thin section, multislice CT and endoscopic ultrasound was demonstrated to have a combined sensitivity for pre-operative detection of insulinomas of 100% [7] (Fig. 3).
Figure 3.
The value of multiphase CT contrast-enhanced imaging in the detection of insulinomas. (a) Twenty-five seconds post contrast, two small hyperattenuating lesions are observed in the head of the pancreas (arrowed). (b) At 40 s there is optimal visualisation of both lesions. (c) At 65 s only one lesion is identified; the second has become isoattenuating.
Gastrinomas
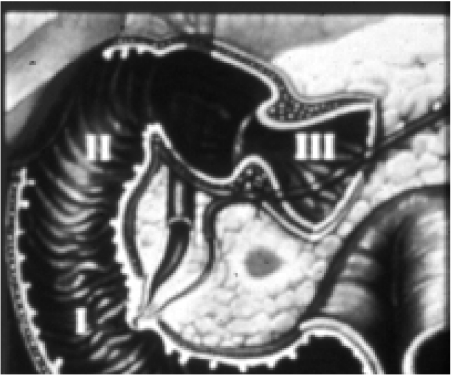
Patients with gastrinomas may present with the Zollinger–Ellison syndrome which links peptic ulcer disease, diarrhoea or abdominal pain with elevated levels of serum gastrin. Ninety percent of gastrinomas occur in what is known as the ‘gastrinoma triangle’ either in the duodenal wall or peri-duodenal tissues or within the head of the pancreas (Fig. 4). Up to 50% may be extra-pancreatic in location and these lesions can be difficult to identify on imaging.
Figure 4.
‘Gastrinoma triangle’. Tumour arises in the pancreatic head, duodenal wall or in the peri-pancreatic tissues.
These tumours are frequently small, between 3 and 5 mm in diameter (40% are less than 1 cm) and often multiple. Fifty to sixty percent of tumours are malignant with metastatic involvement of adjacent lymph nodes and the liver. Endoscopic ultrasound has a high sensitivity in the detection of intra-pancreatic gastrinomas of between 80 and 94% [8, 9], but has a lower sensitivity for duodenal tumours and extra-pancreatic lesion of approximately 50% with an overall sensitivity of approximately 70% [8, 15] (Fig. 5). In patients with clinical evidence of Zollinger–Ellison syndrome, particular attention should be directed to the duodenal wall. In one study, initial endoscopy revealed tumours in the duodenal wall or stomach in 11% of patients presenting with a clinical diagnosis of gastrinoma [9].
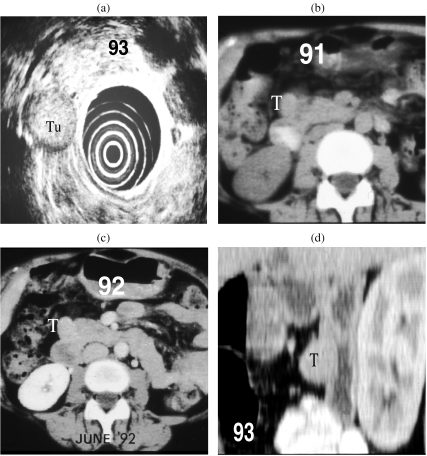
Figure 5.
(a) A well defined mass (Tn) of mid echogenicity seen lateral to the head of the pancreas on EUS in a patient with biochemical evidence of Zollinger–Ellison syndrome. (b, c) CT scans in 91 and 92 had failed to detect the tumour (T) but on repeat scanning (d) the lesion was clearly seen in the duodenal wall on a reconstructed sagittal view.
Gastric carcinoids may arise in association with pancreatic or peri-pancreatic gastrinomas in the context of multiple endocrine neoplasia syndrome (MEN1) and approximately 30% of patients with MEN1 will have gastric carcinoid tumours [16] (Fig. 6). MEN1 is a rare autodominant disorder with many complex endocrine and non-endocrine manifestations. Patients may present with hyperthyroidism, islet cell tumours and pituitary tumours—predominantly prolactinomas and growth hormone releasing tumours. Adreno-cortical tumours may occur as may skin lesions such as collagenomas and lipomas [17].
Figure 6.
(a) Small superficial gastric carcinoid tumour (arrow). (b) Large centrally cystic malignant gastric carcinoid.
CT is typically the primary investigative modality in patients suspected of Zollinger–Ellison syndrome or MEN1 because of the high rate of malignancy and the superior sensitivity of CT for metastatic disease.
Somatostatin receptor scanning is also valuable in this context with a high sensitivity for gastrinomas of between 74 and 87% [8]. The sensitivity for insulinomas is less, as only approximately 50% of insulinomas express somatostatin receptor activity.
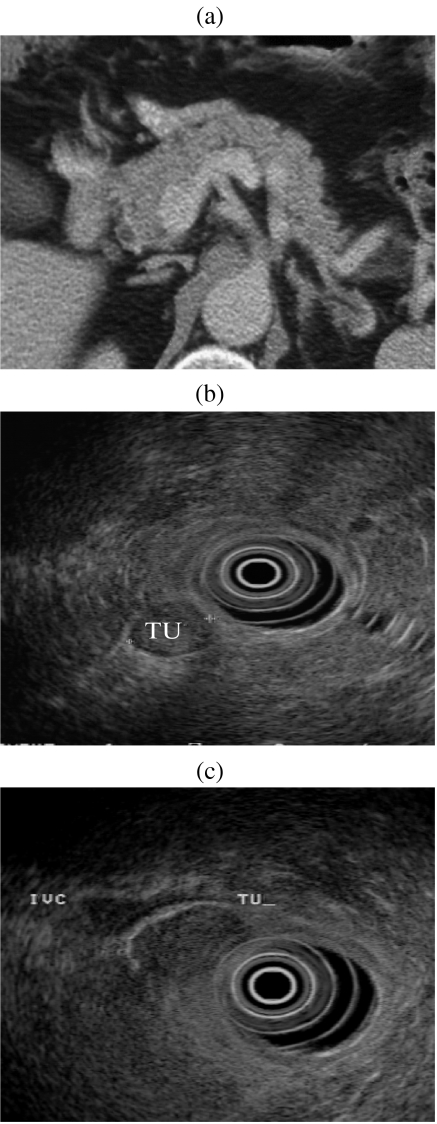
We use EUS as a second-line investigation after CT scanning in this group of patients. Not uncommonly, EUS will detect lesions in the duodenal wall or peri-duodenal extra-pancreatic tissues which prior CT has failed to identify. Retrospective review of the CT scan or repeat CT with negative duodenal contrast (water distension) and biphasic scanning will frequently demonstrate these extra-pancreatic lesions in retrospect (Fig. 7).
Figure 7.
(a) CT scan of pancreas reported as normal in a patient with biochemical evidence of a gastrinoma. (b, c) EUS demonstrates two small tumour nodules inferior to pancreatic head. (d, e) Review of the CT reveals two small enhancing nodules corresponding to the EUS appearance. Histology reveals two foci of malignant gastrinoma in peri-pancreatic nodes.
Role of EUS in differential diagnosis and staging
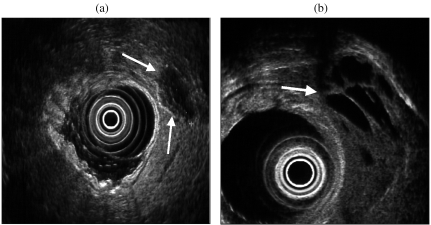
Patients who are found to have a pancreatic mass on other imaging tests are frequently referred for EUS for staging. There are certain features on EUS which suggest that a tumour may have an islet cell origin as compared to pancreatic adenocarcinoma. Demonstration of cystic change within the lesion or calcification may be an indication that the lesion is other than a typical adenocarcinoma (Fig. 8). A recent report highlighted certain EUS features which may allow a differentiation between benign and malignant non-functioning islet cell tumours, although the reported series is small [18]. Malignant lesions tend to be larger with a mean diameter of greater than 4 cm (Fig. 9). They are frequently more heterogeneous in echotexture with irregular central echogenicity, and may displace or obstruct the main pancreatic duct. However, the specificity of using morphological features alone to differentiate pancreatic mass lesions is relatively low: 53% [19].
Figure 8.
(a) Non-functioning islet cell tumour with peripheral calcification. (b) Cystic change within an insulinoma.
Figure 9.
Irregular peripheral margins in a malignant insulinoma.
The accuracy of EUS staging prior to surgery has been addressed in several studies, particularly in relation to vascular invasion. Tio et al. reported an overall accuracy of 83.6% of local tumour staging considering size, invasion of local organs and major vessels [20]. There are specific signs which may indicate vascular invasion with variable accuracy—proximity of the mass to a vessel (73%), loss of the interface between the mass and vessel (78%) and irregularity of the venous wall (87%) [21]. However, the sensitivity for superior mesenteric vein invasion is low (17%), which reduces the overall sensitivity of this technique to 47%.
Establishing a firm diagnosis of islet cell tumour pre-operatively may allow for a more aggressive surgical approach in these tumours. A surgical series of 31 patients with large or malignant islet cell tumours of greater than 4 cm in diameter reported an overall 5-year survival of 75% following aggressive surgery with vascular reconstruction and resection of any involved adjacent organs or metastases. These results are clearly superior to those reported for pancreatic adenocarcinoma [22].
EUS-guided biopsy
The development of curvilinear ultrasound endoscopes has enabled the development of transmural biopsy techniques, guided by EUS. The needle passage through the gastric or duodenal wall into a lesion can be clearly monitored by EUS and fine needle aspiration (FNA) biopsy obtained. A direct approach to the lesion through the gastric or duodenal wall reduces the potential risk of transperitoneal spread of tumour (Fig. 10). Voss et al. reported the results of EUS fine needle aspiration biopsy in 90 patients presenting with a pancreatic mass (adenocarcinoma n=59, neuroendocrine tumours n=15). Diagnostic accuracy of FNA was higher in adenocarcinomas (81%) as compared to neuroendocrine tumours (46.7%) [23]. There are technical difficulties due to problems in positioning, particularly in relation to the pancreatic head and uncinate process. Some tumours may have a very dense fibrotic stroma and are very difficult to penetrate, and in these the FNA biopsy is frequently negative. Approximately 50% of biopsies in the neuroendocrine tumours were false-negative or inadequate in this study. In many of these, the specimen was haemorrhagic and unsuitable for diagnosis.
Figure 10.
(a) Endoscope positioned in the stomach for transgastric wall puncture of a small pancreatic mass. (b) Needle tip (arrow) visualised within the mass.
Experience with this technique is increasing. A recent study included 58 patients with a pancreatic mass in whom CT-guided biopsy was negative. In these patients, EUS FNA had 90% sensitivity for malignancy, 50% specificity for benign disease and 84% accuracy [24]. Similar results have been reported in a further study of 179 EUS FNAs, 91% of which were pancreatic lesions, where an overall sensitivity of 81.7% was found with a false negative rate due to inadequate sampling of 13.2% [25]. Although the results for pancreatic adenocarcinoma are very promising, the final role of EUS-directed biopsy in patients with suspected islet cell tumour has not yet been established.
Conclusion
In summary, endoscopic ultrasound has a high sensitivity in patients with insulinoma and should be regarded as a primary diagnostic tool. In patients with gastrinoma where there is a higher possibility of malignancy, CT should be used as the first-line investigation and EUS reserved as a second-line investigation in patients who are CT negative. All such patients should also undergo endoscopy to detect small gastric wall duodenal tumours. Where there is strong suspicion of a functioning tumour but other tests are negative, EUS may contribute useful information.
EUS may also provide valuable information regarding resectability in larger non-functioning tumours. The role of EUS-directed FNA biopsy for pre-operative diagnosis in these tumours shows promise but has not yet been firmly established.
References
- 1.Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and periancreatic neuroendocrine tumours: review of 125 patients. J Gastrointest Surg. 1998;2:472–82. [PubMed] [Google Scholar]
- 2.King CM, Reznek RH, Dacie JE, Wass JA. Imaging islet cell tumours. Clin Radiol. 1994;49:295–303. doi: 10.1016/s0009-9260(05)81790-8. [DOI] [PubMed] [Google Scholar]
- 3.Gunther RW, Klose KJ, Ruckert K, Beyer J, Kuhn FP, Klotter HJ. Localization of small islet-cell tumors. Preoperative and intra-operative ultrasound, computed tomography, arteriography, digital subtraction angiography, and pancreatic venous sampling. Gastrointest Radiol. 1985;10:145–52. doi: 10.1007/BF01893089. [DOI] [PubMed] [Google Scholar]
- 4.Rösch T, Lightdale CJ, Botet JF, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721–6. doi: 10.1056/NEJM199206253262601. [DOI] [PubMed] [Google Scholar]
- 5.Pitre J, Soubrane O, Palazzo L, Chapuis Y. Endoscopic ultrasonography for the preoperative localization of insulinomas. Pancreas. 1996;13:55–60. doi: 10.1097/00006676-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher B, Lubkay HJ, Frieling T, Strohmeyer G, Starke AA. Prospective study on detection of insulinomas by endoscopic ultrasonography. Endoscopy. 1996;28:273–6. doi: 10.1055/s-2007-1005452. [DOI] [PubMed] [Google Scholar]
- 7.Gouya H, Vignaux O, Augui J, et al. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. Am J Roentgenol. 2003;181:987–92. doi: 10.2214/ajr.181.4.1810987. [DOI] [PubMed] [Google Scholar]
- 8.Zimmer T, Scherübl H, Faiss S, Stölzel U, Riecken E-O, Wiedenmann B. Endoscopic ultrasonography of neuroendocrine tumours. Digestion. 2000;62(Suppl 1):45–50. doi: 10.1159/000051855. [DOI] [PubMed] [Google Scholar]
- 9.Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumours of the pancreas. Am J Gastroenterol. 2000;95:2271–7. doi: 10.1111/j.1572-0241.2000.02480.x. [DOI] [PubMed] [Google Scholar]
- 10.Ardengh JC, Rosenbaum P, Ganc AJ, et al. Role of EUS in the preoperative localization of insulinomas compared with spiral CT. Gastrointest Endosc. 2000;51:552–5. doi: 10.1016/s0016-5107(00)70288-4. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216:163–71. doi: 10.1148/radiology.216.1.r00jl26163. [DOI] [PubMed] [Google Scholar]
- 12.King AD, Ko GT, Yeung VT, Chow CC, Griffith J, Cockram CS. Dual phase spiral CT in the detection of small insulinomas of the pancreas. Br J Radiol. 1998;71:20–3. doi: 10.1259/bjr.71.841.9534694. [DOI] [PubMed] [Google Scholar]
- 13.Van Hoe L, Gryspeerdt S, Marchal G, Baert AL, Mertens L. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. Am J Roentgenol. 1995;165:1437–9. doi: 10.2214/ajr.165.6.7484581. [DOI] [PubMed] [Google Scholar]
- 14.Fidler JL, Fletcher JG, Reading CC, Andrews JC, Thompson GB, Grant CS, Service FJ. Preoperative detection of pancreatic insulinomas on multiphasic helical CT. Am J Roentgenol. 2003;181:775–80. doi: 10.2214/ajr.181.3.1810775. [DOI] [PubMed] [Google Scholar]
- 15.Ruszniewski P, Amouyal P, Amouyal G, et al. Localisation of gastrinomas by endoscopic ultrasonography in patients with Zollinger–Ellison syndrome. Surgery. 1995;117:629–35. doi: 10.1016/s0039-6060(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 16.Binstock AJ, Johnson CD, Stephens DH, Lloyd RV, Fletcher JG. Carcinoid tumors of the stomach: a clinical and radiographic study. Am J Roentgenol. 2001;176:947–51. doi: 10.2214/ajr.176.4.1760947. [DOI] [PubMed] [Google Scholar]
- 17.Zarnegar R, Brunaud L, Clark OH. Multiple endocrine neoplasia type I. Review. Curr Treat Options Oncol. 2002;3:335–48. doi: 10.1007/s11864-002-0033-0. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama M, Nobutsugu A, Yumi I, et al. Differential diagnosis of benign versus malignant non-functioning islet cell tumours of the pancreas: the roles of EUS and ERCP. Gastrointest Endosc. 2002;55:115–9. doi: 10.1067/mge.2002.119604. [DOI] [PubMed] [Google Scholar]
- 19.Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher-Ravens A, Knofel WT, Jackle S, Soehendra N. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35:1221–8. doi: 10.1080/003655200750056736. [DOI] [PubMed] [Google Scholar]
- 20.Tio TL, Sie LH, Kallumaris G, et al. Staging of ampullary and pancreatic carcinoma: comparison between endosonography and surgery. Gastrointest Endosc. 1996;44:706–13. doi: 10.1016/s0016-5107(96)70056-1. [DOI] [PubMed] [Google Scholar]
- 21.Brugge WR, Lee MJ, Kelsey PB, Schapiro RH, Warshaw AL. The use of EUS to diagnose malignant portal venous system invasion by pancreatic cancer. Gastrointest Endosc. 1996;43:561–7. doi: 10.1016/s0016-5107(96)70191-8. [DOI] [PubMed] [Google Scholar]
- 22.Hellman P, Andersson M, Rastad J, et al. Surgical strategy for large or malignant endocrine pancreatic tumors. World J Surg. 2000;24:1353–60. doi: 10.1007/s002680010224. [DOI] [PubMed] [Google Scholar]
- 23.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 25.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M.D. Anderson Cancer Center experience. Cancer. 2002;96:174–80. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]