Abstract
With expenditure on imaging patients with cancer set to increase in line with rising cancer prevalence, there is a need to demonstrate the cost-effectiveness of advanced cancer imaging techniques. Cost-effectiveness studies aim to quantify the cost of providing a service relative to the amount of desirable outcome gained, such as improvements in patient survival. Yet, the impact of imaging on the survival of patients with cancer is small compared to the impact of treatment and is therefore hard to measure directly. Hence, techniques such as decision-tree analysis, that model the impact of imaging on survival, are increasingly used for cost-effectiveness evaluations. Using such techniques, imaging strategies that utilise computed tomography, magnetic resonance imaging and positron emission tomography have been shown to be more cost-effective than non-imaging approaches for the management of certain cancers including lung, prostate and lymphoma. There is stronger evidence to support the cost-effectiveness of advanced cancer imaging for diagnosis, staging and monitoring therapy than for screening. The results of cost-effectiveness evaluations are not directly transferable between countries or tumour types and hence more studies are needed. As many of the techniques developed to assess the evidence base for therapeutic modalities are not readily applicable to diagnostic tests, cancer imaging specialists need to define the methods for health technology assessment that are most appropriate to their speciality.
Keywords: Diagnostic imaging, neoplasms, cost-effectiveness, decision trees, evidence-based medicine
Introduction
Advanced diagnostic imaging technologies such at computed tomography (CT), magnetic resonance imaging (MRI), single photon emission computerised tomography (SPECT) and positron emission tomography (PET) are increasingly advocated for optimal care of patients with cancer. The rising prevalence of cancer within an ageing population means that considerable growth in expenditure on these imaging techniques can be anticipated. With the inevitable constraints of fixed budgets for health care, governments and other purchasers of health care have a responsibility to ensure that there is good evidence for the safety and efficacy of the procedures purchased on behalf of patients. Within this setting of evidence-based medicine, it is pertinent to ask whether cancer imaging provides ‘value for money’. This article summarises the available methods for assessing the cost-effectiveness of diagnostic imaging modalities and considers the available evidence supporting the cost-effectiveness of various aspects of cancer imaging, particularly the use of CT, MRI, SPECT and PET as compared to simpler diagnostic tests and non-imaging approaches.
What is cost-effectiveness?
Cost-effectiveness studies aim to quantify the cost of providing a service relative to the amount of desirable outcome gained. However, the conditions under which a service is considered cost-effective will vary considerably depending on one’s perspective. A ‘social’ perspective tends to take into account all of the costs and benefits of the alternatives considered, regardless of which parties incur the costs and receive the benefits. Such studies are often described as ‘economic evaluations’. Other studies might be concerned only with the subset of costs and consequences affecting the party that has commissioned the evaluation. These studies are known as ‘financial evaluations’.
Cost
Measuring the costs of medical interventions can be quite difficult, especially for the more inclusive, economic evaluations. It is important that cost estimates include not only the costs of diagnostic tests but also the costs of subsequent clinical management resulting from the test results. These downstream costs are often considerably greater than the diagnostic costs, especially if they include surgery or chemotherapy. The term ‘opportunity cost’ describes the value of benefit foregone by undertaking a new activity. Such opportunities forgone may also be non-diagnostic and in a fixed-budget setting, an increase in expenditure on diagnosis may dictate reduced expenditure on another activity, such as treatment.
Effectiveness and the measurement of outcomes
The effectiveness of a diagnostic test can be considered at a number of different levels ranging from safety and technical performance, through diagnostic performance (e.g. sensitivity, specificity) to therapeutic impact (e.g. changes in clinical management) and health impact [1]. Health impact assessments provide the highest-level assessment of effectiveness and aim to determine the effect that management changes induced by a diagnostic test have upon ultimate health outcomes such as survival, quality-of-life and quality-adjusted survival, measured in quality-adjusted life-years (QALYs). Demonstrating the impact of imaging on ultimate outcomes can be extremely difficult because many steps in the care process intervene between diagnostic imaging and ultimate outcome and the statistical variance that arises at each of these steps obscures the effects of imaging [2]. However, evidence of improved proximal outcomes, such as change in diagnosis or management, should be complimented by evidence of treatment efficacy or quantitative modelling of likely ultimate outcomes using techniques such as decision-tree analysis (see below).
Marginal analysis and the incremental cost-effectiveness ratio (ICER)
Marginal analysis involves calculating the additional costs and benefits associated with a change of some kind. The cost and effectiveness of an imaging strategy can be compared to an alternative management strategy by using the ICER, defined as:
 |
If the imaging strategy has a greater effectiveness for a lower cost, the imaging strategy is said to ‘dominate’ whereas the alternative strategy is ‘dominant’ if the imaging strategy has greater cost for lower effectiveness. In both cases the ICER is negative. If the imaging strategy has improved effectiveness but with greater cost, the ICER can be used to help decide whether the additional benefit is worth the additional cost. There is no generally accepted ICER value beyond which health sector interventions are considered ‘acceptable’ and such judgements may vary considerably between different countries and health care systems.
How is cost-effectiveness assessed?
Clinical consensus has been used widely to establish guidelines for the effective use of diagnostic imaging in several countries but confers only low level evidence for cost-effectiveness. More objective and quantitative evidence of cost-effectiveness can be provided either by case-tracking methods or by decision modelling.
Case-tracking studies focus on a series of patients who undergo a particular diagnostic test and individual patients are tracked to determine the costs and benefits that accrue. Ideally, such studies would have a randomised-controlled design but although randomised-controlled trials (RCTs) are well established in the assessment of therapeutic manoeuvres, such studies present distinct difficulties when applied to diagnostic imaging technologies [2]. A self-controlled study design offers an alternative in which the clinician is asked to record at the time of referral, the clinical management intended had the imaging modality not been available. Case tracking is then used to determine the actual clinical management that occurred following receipt of the imaging results and compares the actual clinical management to the originally intended plan. Any changes in management can be observed and their costs and benefits assessed.
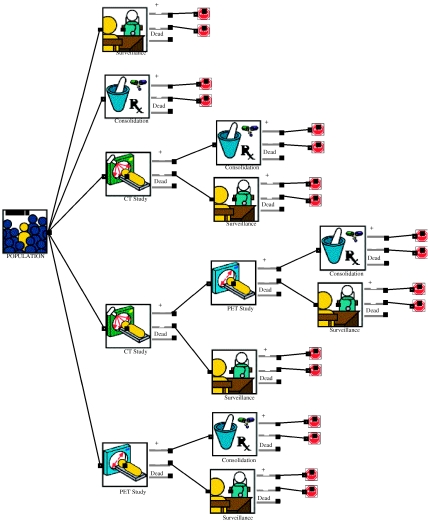
Decision modelling has emerged as a powerful tool for assessing the likely cost-effectiveness of diagnostic imaging strategies when RCTs are either impossible or unavailable. Each management strategy is represented by a horizontal flow chart with branching points at which a decision is made, resulting in a range of possible outcomes (see Fig. 1). The likelihood, cost and value of each outcome associated with all strategies are determined and the average cost and outcome per patient are calculated (e.g. in QALYs) based upon estimates of disease prevalence, diagnostic performance (sensitivity and specificity) of diagnostic tests and costs of diagnostic and therapeutic procedures. Decision modelling studies often incorporate a ‘sensitivity analysis’ to allow for any uncertainty about the input assumptions.
Figure 1.
A decision tree comparing five strategies for clinical management following induction chemotherapy for Hodgkin’s disease based on the study undertaken by the Health Technology Board for Scotland [18]. [Produced using ExtendTM software (Imagine That, Inc., San Jose, USA) with medical imaging blocks from the Crump Institute, UCLA.]
Cost-effectiveness studies of imaging in oncology
Screening
The requirements that need to be fulfilled to render a diagnostic imaging strategy cost-effective for screening are different to those required for effective diagnosis. Firstly, the prevalence of disease within the screened population needs to be sufficiently high. Hence, many screening programs target groups with a higher probability of malignancy. However, even with targeting, the prevalence of cancer amongst those undergoing screening will be considerably lower than amongst patients presenting with clinical symptoms. With low disease prevalence, the specificity of the diagnosis test (i.e. the ability to identify patients without the disease) must be very high to avoid large numbers of false-positive results per cancer case detected. Patients with false-positive results undergo the morbidity of unnecessary assessment tests such as further imaging or biopsy. These additional tests also increase the costs of a screening programme. A further requirement for effective screening is that the curative potential should be improved by early detection.
Screening for breast cancer with biennial mammography for women aged 50–70 years has proven cost-effective in many countries in Europe and in the USA. The ICER compared to surveillance alone is highly variable across nations with decision-tree analyses giving estimates from approximately £1100/life-year gained in the UK to $21 400/life-year in the USA [3, 4]. In contrast to mammography, the ability of the higher cost techniques of CT, MRI and PET to be cost-effective in cancer screening remains to be demonstrated. CT screening for lung cancer has attracted much interest but decision-tree analyses of cost-effectiveness have produced variable results [5]. These studies have been the subject of much debate with ICER estimates based on USA cost structures ranging between $19 000/life-year for a financial evaluation and $116 300/QALY for a full economic evaluation. However, these studies have relied on assumptions about the degree of stage-shift and associated survival benefit from early diagnosis that are yet to be confirmed by on-going clinical trials. A decision-tree analysis of the cost-effectiveness of CT colography in subjects aged 50 years demonstrated an ICER compared to no screening of $24 586/life-year [5]. However, colonoscopy would be more cost-effective at $20 930/life-year. Untargeted whole-body CT screening has not been subject to technology evaluation but has raised concerns about the downstream costs of investigating incidental benign lesions.
With costs exceeding those of CT, demonstrating cost-effective applications for MRI and PET in cancer screening will prove even harder. MRI has been evaluated in screening for breast cancer in women with a genetic preposition but has been found to be expensive, costing EUR13 930 per detected cancer [6]. A study of PET screening for cancer in 3165 asymptomatic individuals found only 36 tumours (1.1%) [7].
Diagnosis
The investigation of the solitary pulmonary nodule (SPN) is perhaps the diagnostic cancer imaging application most thoroughly evaluated in terms of cost-effectiveness. Prior to the availability of CT and PET, management of a SPN could be watchful waiting or immediate biopsy. The choice between these options could be based on an assessment of the prior probability of malignancy estimated from factors such as age and smoking history. A number of cost-effectiveness studies from several countries have compared watchful waiting strategies with the use of CT and/or fluorodeoxyglucose (FDG)–PET in order to characterise a SPN as benign or malignant and so select patients for biopsy.
Compared to watchful waiting, the cost of CT characterisation and subsequent management of an SPN in the USA has recently been estimated to be between $6515 and $10 935/life-year gained [8]. These cost-effectiveness values correspond to prior probabilities of malignancy of 79 and 26%, respectively. Based on Australian costs and data, CT characterisation and subsequent management was estimated to cost AU$16 850 per correctly managed SPN when the prior probability of malignancy was 54% [9]. However, several studies have demonstrated that the addition of FDG–PET to management strategies for SPN produces a further incremental gain in cost-effectiveness, particularly if reserved for those patients for whom CT is indeterminate. Some studies report that use of PET can produce incremental cost savings with no loss of life-expectancy over a wide range of pre-test likelihood for malignancy whilst ICER values of Y218000 ($1557) and EUR3218 per life-year gained have been determined for Japan and Germany, respectively [10–12]. A recent study has indicated that incorporating a quantitative contrast-enhanced CT study into the characterisation of SPNs can potentially enhance the cost-effectiveness of both CT- and PET-based strategies [13].
Staging
Most evaluations of the cost-effectiveness of imaging in cancer staging compare the relative merits of different strategies incorporating one or more advanced imaging techniques and therefore assume that some form of imaging is desirable. Nevertheless there are studies that have shown improved cost-effectiveness for advanced imaging techniques over non-imaging approaches in the staging of prostate and pancreatic cancer.
As yet, it is not universal practice to use MRI for local staging of prostate cancer. Many urologists rely on clinical (rectal) examination, serum prostate specific antigen (PSA) levels and the pathological Gleason score to predict the likelihood of extracapsular spread of tumour. Radical surgery is usually withheld if extracapsular spread is believed to be present. A meta-analysis of the accuracy of MRI in staging prostate cancer has shown that sensitivity and specificity values for the detection of extracapsular spread are highly variable [14]. If the threshold for diagnosing extracapsular tumour is set high, the sensitivity is low (30%) but the specificity is sufficiently high (97%) such that false-positive results are unlikely. Using MRI in this way to decide which patients should undergo radical prostatectomy can potentially reduce the numbers of patients undergoing surgery in the presence of extracapsular spread undetected by conventional means. MRI could also be used similarly to determine the suitability of patients for prostate brachytherapy which is also only appropriate for tumours confined to the prostate.
Jager et al. [15] used decision-tree analysis to compare the costs and quality-adjusted survival associated with strategies that used conventional means or MRI to select patients for radical prostatectomy. The model included the costs and impact on quality-of-life resulting from complications of surgery such as impotence and incontinence. Based on USA cost structures, the MRI strategy was less expensive ($10 568 vs. $11 669 per patient) with little change in quality-adjusted survival (12.53 QALY vs. 12.52 QALY). If the prior probability of extracapsular spread was greater than 39%, the MRI strategy was both less expensive and more effective, producing improvements in quality-of-life. However, if this probability was less than 10%, the likelihood of MRI finding a patient with extracapsular spread was too low to make its use cost-effective. Thus, MRI would prove cost-effective for selected patients depending on the likelihood of extracapsular spread.
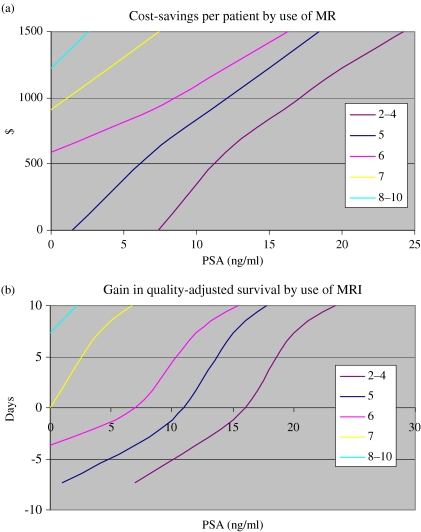
The prior probability of extracapsular spread can be estimated from the PSA level and Gleason score as described by Partin et al. [16]. Based on these estimates and the results of the cost-effectiveness study above, it is possible to generate nomograms that display the cost savings and gains in quality-adjusted survival for given PSA levels and Gleason scores (Fig. 2).
Figure 2.
Likely cost savings (a) and gain in quality-adjusted survival (b) by use of MRI based on PSA and Gleason score (derived from Jager et al. [15] and Partin et al. [16]).
Decision-tree analysis has also been used to assess the cost-effectiveness of advanced imaging techniques in the staging of pancreatic cancer by comparing various imaging strategies with best supportive care [17]. Using USA cost structures, all imaging strategies were more cost-effective than proceeding to surgery without imaging. However, compared to best supportive care, the most cost-effective strategy, CT followed by laparoscopy with laparoscopic ultrasound, achieved an ICER of $87 502, being well above most thresholds for acceptable cost per life-year gained. This finding most likely reflects the limited survival benefit that can be achieved by surgery for pancreatic cancer rather than indicating poor diagnostic performance.
Therapeutic monitoring
There have been few studies evaluating the cost-effectiveness of imaging following cancer therapy. However, the recent assessment of PET undertaken by the Health Technology Board for Scotland included a thorough economic evaluation of CT and PET imaging at the completion of induction chemotherapy for Hodgkin’s disease [18]. The study used a decision-tree analysis that comprised five strategies (see Fig. 1). Two strategies involved no imaging, i.e. immediate consolidation radiotherapy for all patients or surveillance for all patients. In the other strategies patients were allocated to consolidation therapy or surveillance based upon CT alone, FDG–PET alone or PET performed if CT suggested residual disease. With a life-expectancy of 20.8 years and a cost of £4041/patient, CT alone would produce longer life-expectancy and less expenditure than the strategy in which all patients underwent surveillance. However, the model predicted that using CT alone would result in 36% of patients having unnecessary consolidation radiotherapy. This number would be reduced to 4% by use of FDG–PET alone, producing a 0.7 year gain in life-expectancy and a cost saving of £236 per patient.
An application for advanced cancer imaging that could be highly cost-effective is the use of functional imaging to select patients for chemotherapy. One example would be the use of 99mTc methoxyisobutylisonitrile (MIBI) SPECT to identify patients with non-small-cell lung cancers that express p-glycoprotein mediated multidrug resistance (MDR) and so fail to respond to chemotherapy. A previous study indicates that using a threshold tumour-to-background uptake ratio of 2, this technique can identify responders with 100% sensitivity and 80% specificity [19]. The potential cost-effectiveness of such an approach can be estimated by adding an additional strategy to the cost-effectiveness study of chemotherapy in lung cancer commissioned by the UK’s The National Institute of Clinical Excellence (NICE) [20]. In this additional strategy, only patients with tumour-to-background ratios of 2 and above would receive chemotherapy (Paclitaxel 135 mg/m2 and Cisplatin), the remainder receiving best supportive care. Based on a cost for SPECT derived from a further NICE report, Table 1 compares the costs and effectiveness of the three strategies and demonstrates a potential saving of £964 per patient with no loss in life-expectancy which, if extrapolated to a national level, could save in excess of £1.5 million per year. However, further studies are required to confirm the reliability of this application of MIBI SPECT.
Table 1.
The potential improvements in cost-effectiveness produced by using MIBI to select patients with non-small-cell lung cancer for chemotherapy
| Strategy | Costs |
Effectiveness |
Cost-effectiveness |
|||||
|---|---|---|---|---|---|---|---|---|
| Cost/patient | Incremental | Life-years | Incremental | Cost/life-year | ICER | |||
| cost/patient | life-years | |||||||
| BSC | 3210 | — | 0.43 | — | 7408 | — | ||
| All chemotherapy | 6283 | 3073 | 0.78 | 0.35 | 8021 | 8781 | ||
| Selection for chemotherapy based on MIBI | 5319 | 2109 | 0.78 | 0.35 | 6819 | 6026 | ||
MIBI, methoxyisobutylisonitrile imaging; BSC, best supportive care; ICER, incremental cost-effectiveness ratio.
Translating the results of cost-effectiveness studies to other countries
An important limitation of cost-effectiveness studies in imaging is that the results indicating a cost-effective imaging strategy in one country cannot be assumed to apply elsewhere [21]. A major determinant of cost-effectiveness is the cost of imaging relative to the cost of treatments saved by more accurate diagnostic assessment. For example, international variations in the ratio of PET costs to surgical costs have been estimated at between 4 and 30% [22]. Cancer imaging is more likely to prove cost-effective for a broader range of indications in those countries where this ratio is lowest. However, international differences other than cost can also impact upon cost-effectiveness studies, including variations in disease prevalence. These differences may either be in the prevalence of the disease to be detected or in the prevalence of a second disease that can produce false-positive imaging results, thereby reducing the specificity values obtained for an imaging test in a particular country. One example of the latter would be the variable prevalence of granulomatous disease of the lung, a recognised cause for false-positive FDG–PET results in SPNs.
Although the results of case-tracking studies are only applicable to populations with a similar disease prevalence and imaging performance, decision-tree studies that incorporate sensitivity analysis can model the effects that any population differences may have upon cost-effectiveness [21]. However, decision-tree analyses are only useful when the model closely approximates actual clinical practice. Demonstrating that the results of local case-tracking studies are consistent with the findings of decision-tree analysis can usefully validate the model employed.
Summary
There are clear examples in which CT, MRI and PET can be shown to be cost-effective in the management of patients with cancer. The case for the cost-effectiveness of these advanced cancer imaging techniques is stronger for applications in diagnosis, staging and monitoring therapy than for screening. However, the inability to transfer study results between tumour types, or from one country to another, means that many more studies will be required to confirm broad cost-effectiveness of cancer imaging at an international level. With health care resource allocation increasingly made on the basis of evidence of cost-effectiveness, it is essential that cancer imaging specialists engage with the processes of evidence-based medicine as applied to imaging. As many of the techniques developed to assess the evidence base for therapeutic modalities are not readily applicable to diagnostic tests, there is a need to define and develop methods of evaluation that are most appropriate to cancer imaging.
Key points
Demonstrating the cost-effectiveness of imaging technologies is growing in importance as expenditure on imaging patients with cancer is set to increase with rising cancer prevalence.
Imaging strategies that utilise CT, MRI and PET have been shown to be more cost-effective than non-imaging approaches for the management of certain cancers in some countries.
There is stronger evidence to support the cost-effectiveness of advanced cancer imaging techniques for diagnosis, staging and monitoring therapy than for screening.
More studies are needed as the results of cost-effectiveness evaluations are not directly transferable between countries or tumour types.
Cancer imaging specialists need to define the methods for health technology assessment most appropriate to their speciality.
References
- 1.Mackenzie R, Dixon AK. Measuring the effects of imaging: an evaluative framework. Clin Radiol. 1995;50:513–8. doi: 10.1016/s0009-9260(05)83184-8. [DOI] [PubMed] [Google Scholar]
- 2.Sunshine JH, McNeil BJ. Rapid method for rigorous assessment of radiologic imaging technologies. Radiology. 1997;202:549–57. doi: 10.1148/radiology.202.2.9015089. [DOI] [PubMed] [Google Scholar]
- 3.van Ineveld BM, van Oortmarssen GJ, de Konig HJ, Boer R, van der Maas PJ. How cost-effective is breast cancer screening in different EC countries? Eur J Cancer. 1993;29A:1663–8. doi: 10.1016/0959-8049(93)90100-t. [DOI] [PubMed] [Google Scholar]
- 4.Salzmann P, Kerlikowske K, Phillips K. Cost-effectiveness of extending screening mammography guidelines to include women 40 to 49 years of age. Ann Intern Med. 1997;127:955–65. doi: 10.7326/0003-4819-127-11-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Hunink MGM, Gazelle GS. CT screening: a trade-off of risks, benefits, and costs. J Clin Invest. 2002;111:1612–9. doi: 10.1172/JCI18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilanus-Linthorst MM, Obdeijn IM, Bartels KC, de Koning HJ, Oudkerk M. First experiences in screening women at high risk for breast cancer with MR imaging. Breast Cancer Res Treat. 2000;63:53–60. doi: 10.1023/a:1006480106487. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda S, Ide M, Fujii H, et al. Application of positron emission tomography imaging to cancer screening. Br J Cancer. 2000;83:1607–11. doi: 10.1054/bjoc.2000.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003;138:724–35. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- 9.Keith CJ, Miles KA, Griffiths MR, Wong D, Pitman AG, Hicks RJ. Solitary pulmonary nodules: accuracy and cost-effectiveness of sodium iodide FDG–PET using Australian data. Eur J Nucl Med Mol Imaging. 2002;29:1016–23. doi: 10.1007/s00259-002-0833-2. [DOI] [PubMed] [Google Scholar]
- 10.Gambhir SS, Shepherd JE, Shah BD, et al. Analytical decision model for the cost-effective management of solitary pulmonary nodules. J Clin Oncol. 1998;16:2113–25. doi: 10.1200/JCO.1998.16.6.2113. [DOI] [PubMed] [Google Scholar]
- 11.Kosuda S, Ichihara K, Watanabe M, Kobayashi H, Kusano S. Decision-tree sensitivity analysis for cost-effectiveness of chest 2-fluoro-2-D-[(18)F]fluorodeoxyglucose positron emission tomography in patients with pulmonary nodules (non-small cell lung carcinoma) in Japan. Chest. 2000;117:346–53. doi: 10.1378/chest.117.2.346. [DOI] [PubMed] [Google Scholar]
- 12.Dietlein M, Weber K, Gandjour A, et al. Cost-effectiveness of FDG–PET for the management of solitary pulmonary nodules: a decision analysis based on cost reimbursement in Germany. Eur J Nucl Med. 2000;27:1441–56. doi: 10.1007/s002590000324. [DOI] [PubMed] [Google Scholar]
- 13.Comber LA, Keith CJ, Griffiths M, Miles KA. Solitary pulmonary nodules: impact of quantitative contrast-enhanced CT on the cost-effectiveness of FDG–PET. Clin Radiol. 2003;58:706–11. doi: 10.1016/s0009-9260(03)00166-1. [DOI] [PubMed] [Google Scholar]
- 14.Sonnad SS, Langlotz CP, Schwartz JS. Accuracy of MR imaging for staging prostate cancer: a meta-analysis to examine the effect of technologic change. Acad Radiol. 2001;8:149–57. doi: 10.1016/s1076-6332(01)90095-9. [DOI] [PubMed] [Google Scholar]
- 15.Jager GJ, Severens JL, Thornbury JR, et al. Prostate cancer staging: should MR imaging be used?—A decision analytic approach. Radiology. 2000;215:445–51. doi: 10.1148/radiology.215.2.r00ap09445. [DOI] [PubMed] [Google Scholar]
- 16.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localised prostate cancer. J Urol. 1993;150:110–4. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 17.McMahon PM, Halpern EF, Fernandez-del Castillo C, Clark JW, Gazelle GS. Pancreatic cancer: cost-effectiveness of imaging technologies for assessing resectability. Radiology. 2001;221:93–106. doi: 10.1148/radiol.2211001656. [DOI] [PubMed] [Google Scholar]
- 18.Bradbury I, Boynton J, Facey K, Health technology assessment of positron emission tomography (PET) imaging in cancer management: staging non-small cell lung cancer (NSCLC). Consultation Assessment Report, Health Technology Board for Scotland. Glasgow: HTBS
- 19.Shih CM, Hsu WH, Huang WT, Wang JJ, Ho ST, Kao A. Usefulness of chest single photon emission computed tomography with technetium-99m methoxyisobutylisonitrile to predict taxol based chemotherapy response in advanced non-small cell lung cancer. Cancer Lett. 2003;199:99–105. doi: 10.1016/s0304-3835(03)00335-5. [DOI] [PubMed] [Google Scholar]
- 20.Scott DA, Clegg A, Sidhu M, Hewitson P, Waugh N, Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in lung cancer. The National Institute of Clinical Excellence [DOI] [PMC free article] [PubMed]
- 21.Miles KA. An approach to demonstrating cost-effectiveness of diagnostic imaging modalities in Australia illustrated by positron emission tomography. Australas Radiol. 2001;45:9–18. doi: 10.1046/j.1440-1673.2001.00865.x. [DOI] [PubMed] [Google Scholar]
- 22.Miles KA, Connely LB. Cost-effectiveness studies of PET in oncology. In: Oehr P, Biersack H-J, Coleman RE, editors. PET and PET-CT in Oncology. Heidelberg: Springer; 2003. [Google Scholar]