Abstract
Preoperative imaging with MRI/MRA/MRCP is an accurate non-invasive method for staging cholangiocarcinoma, and determining resectability. It provides information regarding tumor size, extent of bile duct involvement, vascular patency, extrahepatic extension, nodal or distant metastases, and the presence of lobar atrophy. MRCP is better for demonstrating bile ducts distal to the stricture, although with ERCP, therapeutic intervention such as stent placement and biopsy can be performed.
Keywords: MRI, MRCP, cholangiocarcinoma
Introduction
Cholangiocarcinomas are tumors that arise from the bile duct epithelium. They may occur anywhere along the intrahepatic or extrahepatic bile ducts, from the liver to the ampulla of vater [1–5]. Cholangiocarcinoma is a rare tumor that comprises less than 2% of all cancer [6]. Intrahepatic cholangiocarcinoma accounts for 5–30% of all primary malignant hepatic tumors, and is the second most common primary malignant tumor of the liver after hepatocellular carcinoma (HCC) [5, 7].
Etiology
Cholangiocarcinoma is more common in men than women, occurring most frequently between the 6th and 7th decades [1, 5, 8–11]. Most patients have no predisposing risk factors, but the presence of the following risk factors may lead to development of the tumor at a younger age [9, 12]: primary sclerosing cholangitis (PSC) (5–15% lifetime risk); choledochal cysts (5% will transform and risk increases with age); Caroli disease (7% lifetime risk); hepatolithiasis; chronic intraductal stones; bile duct adenoma; biliary papillomatosis; Clonorchis sinensis infection; and Thorotrast (thorium dioxide) exposure [2, 12–14]. The occurrence of cholangiocarcinoma in association with chronic inflammatory conditions suggests that inflammation and glandular regeneration may be the precursors to carcinoma [15].
With liver transplantation resulting in reduced mortality from hepatic failure, cholangiocarcinoma has become a leading cause of death in patients with PSC [12, 16, 17]. The occurrence of cholangiocarcinoma has not been shown to relate to the duration or histologic stage of PSC; both diseases may be diagnosed simultaneously, and cholangiocarcinoma may occur before PSC progresses to cirrhosis [12, 17]. Due to the strong association between PSC and ulcerative colitis (UC), it has been suggested that the incidence of cholangiocarcinoma is higher in patients with PSC associated with UC [12, 17].
Classification and pathology
Anatomically, cholangiocarcinomas are classified into three broad groups: (1) intrahepatic; (2) perihilar; and (3) distal extrahepatic [18, 19]. These categories correlate with the anatomic distribution of the tumor and imply preferred treatment. More detailed classification systems have been used whereby the intrahepatic cholangiocarcinomas have been classified into four types [5]: peripheral (intrahepatic); hilar or Klatskin tumor [20] (involves bifurcation of common hepatic duct); hepatic duct (involves a major hepatic duct near the liver hilum); and intraductal (pure intraductal growth). In this review, we will use the former three group classification; intrahepatic cholangiocarcinoma will indicate the peripheral form, and perihilar cholangiocarcinoma will encompass the hilar and hepatic duct forms.
Intrahepatic cholangiocarcinoma arises from small intrahepatic bile duct branches and invades adjacent liver parenchyma [1, 7, 9, 14, 15, 19]. Perihilar cholangiocarcinoma or Klatskin tumor is the most common type and accounts for 50–60% of tumors. The distal extrahepatic type includes tumors that arise from extrahepatic ducts from the level of the upper border of the pancreas to the ampulla of vater [8, 12]. The intrahepatic and distal extrahepatic types comprise 20–25% of cholangiocarcinomas each [14]. Fewer than 10% of patients have multifocal or diffuse involvement of the biliary tree [9, 14, 19].
Macroscopically, three types of cholangiocarcinoma growth have been described [21, 22]: (1) mass-forming (exophytic) type, which results in a definite mass in the liver parenchyma (Fig. 1); (2) infiltrating (periductal) type, which extends longitudinally along the bile duct, often resulting in dilatation of the peripheral ducts (Fig. 2), and is either nodular or diffusely infiltrating [12]; and (3) polypoidal (intraductal) growth type, which proliferates towards the lumen of the bile duct in the form of papillae or tumor thrombus (Fig. 3). Intrahepatic cholangiocarcinomas are typically mass-forming, perihilar and extrahepatic cholangiocarcinomas are mostly infiltrating, and any of the cholangiocarcinomas may rarely have a polypoidal growth pattern. Combined cholangiocarcinoma encompasses more than one growth type, and is more commonly seen with intrahepatic tumors [22].
Figure 1.
Intrahepatic cholangiocarcinoma in a 35-year-old female who presented with right upper quadrant abdominal pain and no prior medical history. There is a large lobulated mass (arrow) in the right lobe of the liver, which, compared to liver parenchyma, is hypointense on the (a) axial T1-weighted (T1W) in-phase spoiled gradient-echo (SGRE) image, and mildly hyperintense on the (b) axial T2-weighted (T2W) fast spin echo (FSE) with fat saturation image. The mass shows heterogeneous rim enhancement on the (c) arterial-dominant phase gadolinium-enhanced axial T1W 3D SGRE image with fat saturation, and fills in heterogeneously on the (d) delayed (15 min) gadolinium-enhanced axial T1W SGRE image. A presumed metastatic nodule (white open arrow) is seen in the lateral segment of the left lobe on an (e) axial T2W FSE with fat suppression image. CT guided biopsy of the dominant mass revealed a cholangiocarcinoma.
Figure 2.
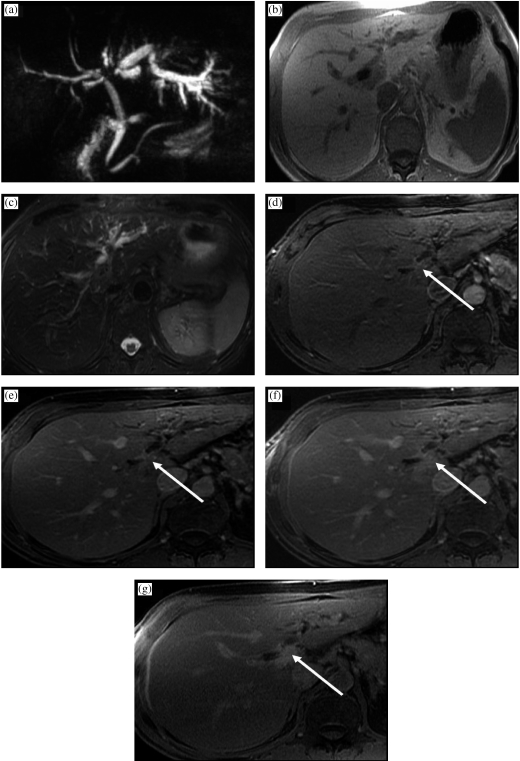
Perihilar cholangiocarcinoma (Klatskin tumor) in a 62-year-old female presenting with painless jaundice. There is mass (arrow) at the junction of the main right and left hepatic ducts. The mass is seen on (a) an MRCP image as a focal stricture involving the duct bifurcation with dilatation of the intrahepatic ducts, left more than right, due to the presence of a right intrabiliary stent. In (b) T1W in-phase SGRE, the mass is hypointense relative to liver parenchyma, and mildly hyperintense on (c) T2W FSE with fat suppression. A susceptibility artifact from the right intrahepatic stent is demonstrated (asterisk). The mass enhances progressively (d–g) following gadolinium administration: in (d) the arterial-dominant phase, (e) portal-venous phase, (f) interstitial phase (2 min post-gadolinium), and is best depicted on (g) the 15 min post-gadolinium image as a hyperintense mass relative to adjacent liver parenchyma.
Figure 3.
Intraductal cholangiocarcinoma in a 70-year-old male who presented with jaundice. An intraductal mass was found on ERCP. There is a minimally enhancing mass within the right main hepatic duct (arrow) on the (a) axial gadolinium-enhanced T1 SGRE image. The mass extends to the bifurcation of the ducts, and has resulted in intrahepatic ductal dilatation. The intraductal cholangiocarcinoma is seen as a filling defect (arrow) on the (b) coronal T2W SSFSE MRCP images. Cytology and brushings revealed a cholangiocarcinoma.
More than 90% of cholangiocarcinomas are adenocarcinomas. Other tumor types include squamous cell carcinoma, adenosquamous carcinoma, small cell carcinoma, and undifferentiated types. The histological grade of tumors varies from well-differentiated to undifferentiated [19]. Most tumors consist of clusters of cells, surrounded by desmoplastic stroma, which can be extensive. The latter feature makes it difficult to distinguish between reactive tissue and well-differentiated cholangiocarcinoma [19, 23]. Furthermore, intrahepatic cholangiocarcinomas may be confused with metastatic scirrhous carcinoma of the breast or pancreas on liver biopsy [24]. Therefore, a primary adenocarcinoma as a source for metastases should be excluded when considering an intrahepatic cholangiocarcinoma [14, 25–27].
Diagnosis, treatment, and prognosis
Patients with perihilar cholangiocarcinoma (Klatskin tumor) usually present with signs and symptoms related to bile duct obstruction. Jaundice is often the first sign; if not, it begins shortly after the onset of right upper quadrant pain. Less common presenting symptoms include pruritus, fatigue, anorexia, and weight loss [12]. Conversely, intrahepatic cholangiocarcinoma does not cause jaundice and patients usually present with abdominal pain, weight loss, or a palpable mass later in the course of the disease [3, 7, 12]. Advanced disease may result in massive ascites due to diffuse hepatic involvement or carcinomatosis [27].
Liver function tests often show an obstructive picture with elevated bilirubin, alkaline phosphatase, and gamma-glutamyltransferase. Aminotransferases are relatively normal or minimally elevated, but may be markedly raised in acute obstruction or cholangitis [12, 14].
Carbohydrate antigen 19-9 (CA19-9) levels with or without carcinoembryonic antigen (CEA) levels may be elevated in patients with cholangiocarcinoma, and can be used for screening high-risk patients for the development of cholangiocarcinoma [28, 29]. However, not all patients with cholangiocarcinoma have elevated CA19-9, and the specificity of this marker is low [28].
Tissue diagnosis of cholangiocarcinoma can be established using percutaneous fine-needle aspiration (FNA), brush and scrape biopsy, and cytological examination of bile [12]. Bile obtained from a percutaneous catheter will be positive for malignant cells in approximately 30% of cases. This yield can be improved to 40% by brush cytologic techniques and to 67% by percutaneous FNA. Nevertheless, as many as one-third of patients with cholangiocarcinoma have negative biopsy or cytology results. If surgery is contemplated, a preoperative tissue diagnosis is not essential, and prolonged efforts to obtain tissue diagnosis are only indicated if the patient is not a surgical candidate [12].
The prognosis of cholangiocarcinoma is poor with an overall 5-year survival rate of 1% [8, 30], and a median survival of approximately 6 months without treatment [4]. Radiation and chemotherapy have shown no benefit, with the only possibility of cure being complete surgical resection or transplantation [8, 12, 13].
With newer surgical techniques, the 5-year survival rate has improved to 11–31% [2, 8, 12]. The likelihood of unresectability is higher for perihilar than intrahepatic or distal extrahepatic cholangiocarcinoma [12]; the resectability rate of hilar cholangiocarcinoma is usually 15–20%, and the surgical mortality rate is high, usually 20–30% [2, 30], even in specialized centers [31, 32]. The reported 5-year survival rates for resected intrahepatic, perihilar, and distal extrahepatic tumors are 44, 11, and 28%, with median survival rates of 26, 19, and 22 months, respectively [18]. Preoperative staging is therefore crucial to prevent unnecessary high-risk surgery. Palliative surgery is often performed to relieve symptoms of obstruction. Currently, percutaneous and endoscopic palliative techniques are commonly used for biliary drainage.
Staging
Tumor staging is performed according to the tumor, node, metastasis (TNM) classification of the American Joint Commission on Cancer (Table 1).
Table 1.
TNM staging classification of cholangiocarcinoma
| TNM classification | |
|---|---|
| of extrahepatic bile | |
| ducts [61] | |
| T stage | Primary tumor |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Ductal wall involvement |
| T2 | Tumor invades beyond the wall of the bile duct |
| T3 | Tumor invades the liver, gallbladder, pancreas, and/or unilateral tributaries of the portal vein (right or left) |
| or hepatic artery (right or left) | |
| T4 | Tumor invades any of the following: main portal vein or its tributaries bilaterally, common hepatic artery, |
| or other adjacent structures, e.g. colon, stomach, duodenum, abdominal wall | |
| N stage | Regional nodes |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis in the hepatoduodenal ligament including: cystic duct, pericholedochal, |
| and hepatic hilar nodes | |
| M stage | Distant metastasis |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Staging | |
| Stage 0 | Tis, N0, M0 |
| Stage IA | T1, N0, M0 |
| Stage IB | T2, N0, M0 |
| Stage IIA | T3, N0, M0 |
| Stage IIB | T1, T2, T3, N1, M0 |
| Stage III | T4, any N, M0 |
| Stage IV | Any T, any N, M1 |
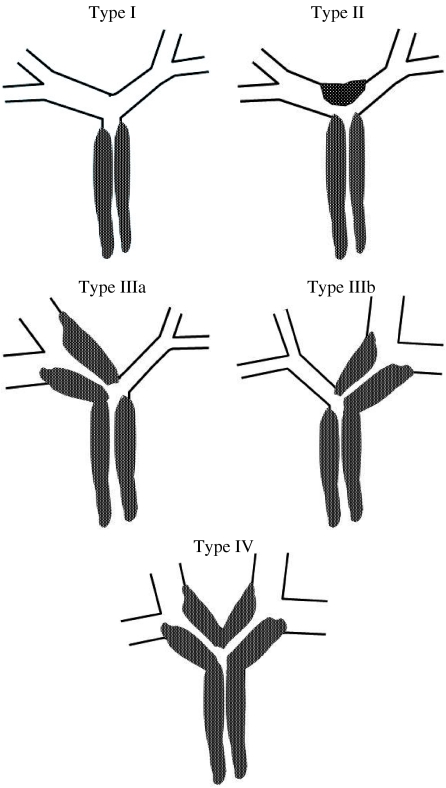
Perihilar cholangiocarcinoma is further classified according to its anatomic location and longitudinal extension along the bile ducts using the Bismuth–Corlette scheme (Fig. 4) [31, 33]. This classification has therapeutic and prognostic implications, and is as follows:
Type I: tumor confined to common hepatic duct (CHD), obstructing the duct within 2 cm of the hilum.
Type II: tumor of the CHD bifurcation involving both main right and left hepatic ducts, and causing obstruction at the hilum with no communication between the main right and left hepatic ducts.
Type IIIa and IIIb: tumors extending into right and left secondary (second order) intrahepatic ducts, respectively, with absence of ductal obstruction on the contralateral side.
Type IV: tumor involves the secondary and tertiary intrahepatic ducts in both lobes causing bilateral obstruction.
Figure 4.
Schematic diagram of the Bismuth–Corlette classification scheme for perihilar tumors. Type I: tumor confined to CHD duct, obstructing the duct within 2 cm of the hilum. Type II: tumor of the CHD bifurcation involving both main right and left hepatic ducts, and causing obstruction at the hilum with no communication between the main right and left hepatic ducts. Type IIIa and IIIb: tumors extending into right and left secondary intrahepatic ducts, respectively, with absence of ductal obstruction on the contralateral side. Type IV: tumor involves the secondary and tertiary intrahepatic ducts in both lobes causing bilateral obstruction.
Preoperative assessment of resectability
Once cholangiocarcinoma is suspected, comprehensive staging must be carried out to screen for metastatic disease. Up to 50% of patients are lymph node positive, and 10–20% have peritoneal involvement at presentation [14, 19]. The preoperative screening requires evaluating the local extent of disease, and the presence of distant metastases. Locally, the level of biliary obstruction, intrahepatic tumor spread, vascular involvement, and lobar atrophy/hypertrophy should be assessed [8]. Chest X-ray for lung metastasis, and in some centers, laparoscopy, to exclude peritoneal metastasis in those patients considered resectable on imaging, is performed [14].
Criteria for tumor unresectability include [4, 8–10, 31, 34]:
Infiltration beyond the second order bile duct branches in both lobes of the liver.
Invasion of major vessels including the main portal vein or main hepatic artery (Fig. 5), both right and left main branches of the portal vein, involvement of the main portal vein branch in one lobe combined with involvement of the main hepatic artery in the contralateral lobe.
A combination of extensive vascular involvement in one lobe and bile duct involvement to the second order branches in the other lobe.
Lymph node metastases beyond N1 station nodes.
Hepatic or distant metastases.
Figure 5.
Inoperable perihilar cholangiocarcinoma in a 64-year-old female who presented with painless jaundice and had focal stenosis of the common bile duct on ERCP. There is a perihilar mass (arrow), which is hypointense to liver on (a) axial T1W in-phase SGRE, mildly hyperintense on (b) T2W FSE with fat saturation, has little to no arterial enhancement on (c) the arterial-dominant phase gadolinium-enhanced axial T1W 3D SGRE, mild enhancement on the (d) 2 min post-gadolinium image, and the tumor becomes mildly hyperintense to liver on the (e) 15 min post-gadolinium image. The tumor surrounds and narrows the main portal vein (white open arrow), and the right main hepatic artery (black open arrow) on a (f) higher slice. (g) Coronal thick slab T2W single shot fast spin-echo (SSFSE) MRCP image demonstrates long segment narrowing of the common hepatic and proximal common bile duct, with a stent traversing the stenotic segment as well as dilation of the intrahepatic bile ducts. (h) A corresponding minimum intensity projection (Min IP) reformatted image demonstrates the mildly enhancing mass surrounding the narrowed segment of common hepatic duct (curved arrow).
Nodal involvement is most common with perihilar cholangiocarcinoma, usually involving the hepatoduodenal ligament lymph nodes [27]. Regional lymph node involvement (N1) does not make the tumor unresectable, as these nodal metastases are resected along with the primary tumor.
Lobar resection is attempted only if there will be sufficient functioning liver parenchyma left after surgery. To ensure potential survival of the remaining liver, in addition to non-diseased adequate volume parenchyma, the remaining lobe should have an uninvolved main hepatic artery, main portal vein branch, and an uninvolved first order bile duct for anastomosis to a loop of bowel for biliary drainage. Therefore, assessment of the degree of tumor extension in the less involved lobe, and the search for nodal and distant metastasis are crucial.
Imaging
The main role of imaging is to determine the resectability of cholangiocarcinoma. Staging has been performed using a combination of visceral angiography, conventional cholangiography and computed tomography (CT). However, a comprehensive magnetic resonance (MR) protocol combining magnetic resonance angiography (MRA) and magnetic resonance cholangiopancreatography (MRCP) can non-invasively provide all the information obtained by angiography, cholangiography and CT.
Unresectability of cholangiocarcinoma, especially advanced cases, is easily predicted by imaging, but predicting resectability is less accurate [2, 27]. Up to 60% of perihilar cholangiocarcinoma considered resectable preoperatively by CT and cholangiography are unresectable at surgery, because of underestimation of the extent of tumor, invasion of the hepatoduodenal ligament, lymph node metastases, and parenchymal infiltration [27]. A study by Nakeeb et al. [18] reported that 45% of patients with perihilar cholangiocarcinoma were found at exploration to have peritoneal or liver metastasis (15%), or extensive tumor involvement of the porta hepatis (30%), precluding surgical resection.
Magnetic resonance imaging (MRI)
Good quality gadolinium-enhanced MRI with MRCP is the optimal imaging examination for suspected cholangiocarcinoma [14]. It provides information regarding liver and biliary anatomy, local extent of tumor, extent of duct involvement by tumor, degree of vascular involvement, presence of lymph node enlargement, liver metastases, and adjacent organ invasion. The technique of MRI is described below.
Morphology
Intrahepatic cholangiocarcinoma presents as a large solitary liver mass, usually between 1 and 14 cm in diameter, with irregular, lobulated or smooth margins [1–3, 35–39]. It does not typically cause biliary obstruction like Klatskin tumor [38], but satellite lesions are more common with peripheral cholangiocarcinoma, due to its late presentation (Fig. 1). Perihilar and extrahepatic cholangiocarcinomas most commonly demonstrate an infiltrating periductal growth pattern, which appears as moderate irregular thickening of the bile duct wall ( cm), narrowing of the duct lumen at the level of the tumor, usually with asymmetric upstream dilatation of the intrahepatic ducts (Fig. 2) [8]. A nodular infiltrating periductal tumor results in protuberant-shaped end morphology of the bile duct, whereas a diffusely infiltrating periductal growth pattern results in a stretched, narrow-lumen duct. Occasionally, focal stenotic periductal tumor can be difficult to differentiate from the intraluminal type [8]. It is necessary to describe the extent of the infiltrating and exophytic components of the tumor to determine the appropriate treatment.
cm), narrowing of the duct lumen at the level of the tumor, usually with asymmetric upstream dilatation of the intrahepatic ducts (Fig. 2) [8]. A nodular infiltrating periductal tumor results in protuberant-shaped end morphology of the bile duct, whereas a diffusely infiltrating periductal growth pattern results in a stretched, narrow-lumen duct. Occasionally, focal stenotic periductal tumor can be difficult to differentiate from the intraluminal type [8]. It is necessary to describe the extent of the infiltrating and exophytic components of the tumor to determine the appropriate treatment.
Signal intensity and enhancement patterns
Similar signal changes are seen on MRI with intrahepatic, perihilar, and extrahepatic cholangiocarcinomas. Typically, the tumor is hypointense on T1-weighted (T1W), and hyperintense on T2-weighted (T2W) imaging relative to liver parenchyma (Figs 1, 2 and 5) [1–3, 8, 10, 27, 35, 36, 38–40]. The degree of hyperintensity on T2W imaging is variable and has been described as mild, moderate, or marked [2, 3, 10, 27, 36, 38–41]. This variability is mostly due to the amount of fibrosis, necrosis, and mucin within the tumor [2], and is influenced by the subtype of the tumor: well-differentiated adenocarcinoma shows higher signal intensity on T2W imaging compared to the scirrhous subtype, which has more fibrosis, less mucin and necrosis [2, 3, 42]. Occasionally, tumors can be isointense to the liver on T1W and T2W imaging [2, 8, 27, 39, 40, 43].
Cholangiocarcinoma is a hypovascular tumor, and following the intravenous administration of gadolinium chelates, the classic enhancement pattern of intrahepatic cholangiocarcinoma is heterogeneous or homogeneous with progressive and prolonged delayed enhancement [2, 3, 25, 36, 38, 41, 44, 45]. The tumor may become isointense or mildly hyperintense compared to the surrounding liver parenchyma on delayed gadolinium-enhanced imaging (Fig. 1) [46]. Four patterns of enhancement of cholangiocarcinoma have been described on early (30 s), late (1 and 3 min) and delayed (5 min) post-gadolinium imaging [2, 36, 37]:
Early peripheral enhancement with progressive and concentric filling in, which is the most common enhancement pattern.
Early peripheral enhancement with non-filling of the central area of the tumor (central scar).
Progressive and complete enhancement.
Early, marked, and complete enhancement, followed by heterogeneous washout of contrast, usually from the periphery of the tumor; this is the least common enhancement pattern.
The variable enhancement pattern of cholangiocarcinoma is related to the amount and distribution of tumor cells and fibrous tissue in the tumor [37]. Progressive and prolonged enhancement is seen in areas of fibrosis where there is decreased arterial blood supply, and large interstitial spaces [36, 37]. Early enhancement and late phase peripheral washout has been shown to correspond to regions of tumor cells, and reflect hypervascularity and increased perfusion. Occasionally, a thin rim of enhancement is seen around the tumor on late phase gadolinium-enhanced imaging, which reflects decreased arterial inflow and washout in a region of congested liver with dilated sinusoids around the tumor [37].
Incomplete filling of the central areas of the tumor, or the central scar, is occasionally seen on post-gadolinium imaging but no corresponding tissue scar has been found on histology [2, 3, 15, 25, 41, 44, 47]. Such “scars” are hypointense on T1W and T2W imaging and correspond to areas of fibrosis, hyalinization and/or coagulation necrosis [2, 37]. Although they do not enhance on late and delayed post-gadolinium imaging, ultra-delayed images may demonstrate enhancement within the scar, due to its fibrous content [36, 45].
The pattern of peripheral enhancement and delayed filling-in is not specific for cholangiocarcinoma, and a similar enhancement pattern can be seen with metastatic liver tumors [44]. Secondary signs that are more commonly associated with cholangiocarcinoma include bile duct dilation distal to the tumor [44], segmental or lobar atrophy associated with the tumor, vascular encasement [15], central scars, and capsular retraction [2, 3, 25, 41, 47, 48].
The enhancement pattern of perihilar and extrahepatic cholangiocarcinomas is similar to that of intrahepatic cholangiocarcinomas. The tumors are hypovascular and enhance slowly and gradually to a peak on delayed imaging (Figs 2 and 5). Because the tumors are usually smaller and typically infiltrating, they are less heterogeneous than intrahepatic tumors. A small percentage of perihilar cholangiocarcinomas are hypervascular and enhance heterogeneously in the arterial-dominant phase [8]. Satellite nodules are less commonly seen with perihilar cholangiocarcinoma compared with the intrahepatic form [8, 43], probably due to the earlier presentation of perihilar tumors with biliary duct obstruction. The central scar described with intrahepatic cholangiocarcinoma is an unusual finding in perihilar cholangiocarcinoma [8]. Perihilar and extrahepatic cholangiocarcinomas are typically seen as abnormal circumferential extrahepatic bile duct wall thickening and enhancement [38, 39], and are best visualized on images obtained 1–5 min after gadolinium administration. Tumor conspicuity increases with fat suppression, which, together with gadolinium enhancement, acts in a complementary fashion [39].
Duct wall enhancement alone may not be a predictor of tumor involvement, and has been demonstrated in normal subjects [39]. It may result from fibrosis or inflammation secondary to bile duct obstruction, and may be particularly prominent following the placement of a biliary stent [37, 39, 49]. Duct wall thickness 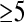 mm has been suggested as a sign of tumor [39]. However, Worawattanakul et al. [39] found that in three of their six patients with circumferential extrahepatic cholangiocarcinomas, the duct wall thickness was
mm has been suggested as a sign of tumor [39]. However, Worawattanakul et al. [39] found that in three of their six patients with circumferential extrahepatic cholangiocarcinomas, the duct wall thickness was  mm. High-grade intrahepatic biliary ductal dilatation distal to the region of minimal duct wall thickening was present in all three patients. The authors suggested that high-grade biliary obstruction, out of proportion to the degree of the duct wall thickening, may be a feature of cholangiocarcinoma. Distal extrahepatic cholangiocarcinomas are frequently mistaken for adenocarcinoma of the pancreatic head [9]. Evaluation with MRCP, as with endoscopic cholangiopancreatography (ERCP), may help demonstrate the biliary origin without involvement of the pancreatic duct [9].
mm. High-grade intrahepatic biliary ductal dilatation distal to the region of minimal duct wall thickening was present in all three patients. The authors suggested that high-grade biliary obstruction, out of proportion to the degree of the duct wall thickening, may be a feature of cholangiocarcinoma. Distal extrahepatic cholangiocarcinomas are frequently mistaken for adenocarcinoma of the pancreatic head [9]. Evaluation with MRCP, as with endoscopic cholangiopancreatography (ERCP), may help demonstrate the biliary origin without involvement of the pancreatic duct [9].
Associated findings
Lobar or segmental atrophy seen in association with the presence of cholangiocarcinoma is thought to be secondary to tumor encasement of the portal venous branches to the tumorous lobe [2] and/or biliary obstruction [39], and can be seen with intrahepatic and perihilar cholangiocarcinoma [4, 9, 39].
Occasionally, a high signal intensity on pre-gadolinium T1W images is seen in the periphery of the segments or lobes in which the tumor is present without portal obstruction, usually around areas of duct dilatation distal to the tumor [50]. These areas show persistent increased enhancement compared to the liver parenchyma in all phases of enhancement following gadolinium administration. No tumor has been shown in these areas on pathology and the delayed enhancement is probably associated with periportal fibrosis secondary to duct dilatation, but the etiology of the high signal intensity on pre-gadolinium T1W images is unclear. It is important not to confuse these areas with foci of metastatic tumor, or the arterial buffer response (transient hepatic intensity difference, THID) caused by portal venous obstruction [50].
Biliary dilatation and duct wall enhancement is commonly seen distal to cholangiocarcinomas [39]. Sometimes, there is abnormal thickening and enhancement of the duct walls, which may represent periductal tumor extension [39], or excessive fibrosis with abundant interstitial space due to cholangitis distal to the tumor [37]. In these cases, it is impossible to accurately determine the degree of periductal tumor extension. Duct wall enhancement with or without wall thickening can also be seen in the presence of intrabiliary stents [39, 49].
Lymph node enlargement is common with cholangiocarcinoma, especially the perihilar type. The portocaval and porta hepatis are the ones most commonly involved [39]. Enlarged lymph nodes are best seen on T2W fat suppressed, and T1W fat suppressed gadolinium-enhanced images [39]. Generally, a short axis diameter greater than 1 cm is used to indicate the increased likelihood of malignant involvement of the node. This criterion is neither sensitive nor specific, and we suggest reporting all visible nodes in the expected regions of metastases, regardless of their size, so they can be examined during surgery. Intrahepatic metastases should be carefully looked for, as they may preclude surgical excision.
MRI has been shown to be useful in detecting peritoneal metastases as small as 1 cm [51, 52]. Peritoneal metastases manifest as peritoneal thickening and enhancement, and are best detected on coronal delayed (5 min) post-gadolinium spoiled gradient-echo (SGRE) images [52]. Using double doses of gadolinium, and oral barium to suppress the signal from bowel lumen, improves the detection of peritoneal metastases [52]. Although MRI has the potential, its accuracy for the detection of peritoneal metastasis from cholangiocarcinoma has not been assessed.
Vascular involvement and MRA
Vascular encasement or compression can be seen with both intrahepatic and perihilar cholangiocarcinoma. Its incidence approaches 50% in hilar tumors [39, 53] and has been described in 82% of peripheral cholangiocarcinomas in a study by Soyer et al. [41]. Unlike HCC, it is more common for cholangiocarcinoma to encase than invade vessels [2, 54], usually the thin-walled portal veins more commonly than the hepatic arteries. Tumor encasement of large vessels such as the hepatic veins, portal veins, or the inferior vena cava without luminal invasion can be well depicted on gradient-echo MRI (Fig. 5) [15]. Encasement of the portal vein or its branches results in an autoregulatory increase in the hepatic arterial supply, which causes a transient increased enhancement of the affected segment or lobe in the hepatic arterial-dominant phase, also known as “transient hepatic intensity difference, THID” [39, 48].
Because of the tendency of cholangiocarcinoma to encase vessels, evaluation of the vasculature is necessary to assess the resectability of the tumor. Conventional angiography is considered more sensitive than MRI for detecting arterial, but not venous involvement [27, 55]. Narrowing of the vessel lumen, abrupt vessel cut-off, and focal irregular indentation are features of vascular invasion on MRA [55]. The reported sensitivity and specificity of MRI assessing involvement of the hepatic venous confluence are 75 and 98%, respectively [56]. With state-of-the-art MRI techniques, such as parallel imaging, high-resolution MRA studies can be obtained, which will improve depiction of arterial involvement (Fig. 5).
MRCP
MRCP plays an important role in the assessment of perihilar cholangiocarcinoma, and in many institutions, it has replaced ERCP and percutaneous transhepatic cholangiography (PTC) for the preoperative staging of the tumor [9, 34]. MRCP can non-invasively evaluate the biliary tree proximal and distal to an obstruction, adding accuracy to the preoperative staging [39, 57–59]. The main advantage of MRCP over ERCP, when assessing perihilar tumors, is its ability to evaluate suprahilar tumor extension which is difficult to assess by ERCP, because of insufficient contrast filling of ducts distal to a constricting tumor [8, 10]. The main drawback of MRCP when compared to ERCP is that it is solely diagnostic.
Perihilar cholangiocarcinoma appears on MRCP as irregular narrowing of the bile duct involved by tumor, with asymmetric upstream dilation of the intrahepatic bile ducts. Information regarding the extent of tumor extension along the bile ducts, whether to one or both lobes, whether the tumor involves first or second order branches, and the presence of concomitant disease, such as hepatolithiasis [57], can be obtained from the MRCP images [10].
The reported sensitivity and specificity of MRCP compared to ERCP for the detection of bile duct malignancy are 81 and 100% compared to 93 and 94%, respectively [4, 58]. MRCP can accurately depict the presence and level of obstruction [34], and has been shown to be more effective than ERCP in delineating the anatomic extent of the cancerous infiltration [34, 57]. Manfredi et al. [8] found that the level and extent of bile duct involvement with cholangiocarcinoma using the Bismuth–Corlette classification was accurately depicted on MRCP in 84% (10 of 12) of their patients.
The combination of parenchymal and vascular information obtained from the T1W, T2W, and gadolinium-enhanced images, and bile duct information obtained from the MRCP images, can be used to accurately stage cholangiocarcinoma. In the study by Yeh et al. [57], the authors found that MRCP was more effective than ERCP in identifying causes of the biliary obstructions and delineating the anatomical extent of the cancerous infiltration. The authors identified 84.6% (22 of 26) of cholangiocarcinomas by the presence of an enhancing mass on delayed gadolinium-enhanced MRI, obtained 5 min after gadolinium injection, but found that in the absence of an enhancing mass, it may be difficult to characterize the etiology of a stricture [60]. Thus, MRCP images alone are inadequate for identifying the cause of biliary strictures as they provide luminal images only, and gadolinium-enhanced imaging is essential for complete evaluation of biliary strictures.
In addition to the standard T2W MRCP images, we have found that using the minimum intensity projection (Min IP) post-processing algorithm to create projectional images of the bile ducts from the 2 min delayed post-gadolinium 3D SGRE images is helpful for additional evaluation of the biliary tree. This allows direct correlation of parenchymal abnormalities with biliary duct abnormalities. With recent technical advances in MRI, in particular the introduction of parallel imaging techniques and newer sequences, high-resolution isotropic 3D MRCP can be obtained with a pixel dimension of 0.7×0.7 mm. Such advances may further improve the accuracy of MRI for staging cholangiocarcinoma.
Summary
In patients with suspected cholangiocarcinoma, a combination of MRI, MRA, and MRCP can be used to evaluate the liver parenchyma, vasculature, and bile ducts. The typical MR signal characteristics of cholangiocarcinoma are hypo- and occasionally isointense signal intensity on T1W, and mild–moderate hyperintense signal intensity on T2W imaging compared to liver parenchyma. Tumors show delayed homogeneous or heterogeneous enhancement, and occasionally early peripheral enhancement of the tumor is seen. The secondary signs of cholangiocarcinoma on MRI, MRA, and MRCP vary depending on the location and stage of the tumor.
Intrahepatic cholangiocarcinoma typically occurs as a large mass, which is difficult to differentiate from a metastatic focus of adenocarcinoma or abscess, and causes biliary duct dilatation peripheral to the tumor.
Perihilar cholangiocarcinoma is the most common subtype, and is typically associated with biliary obstruction, the level and extent of which can be demonstrated on MRCP. This subtype of cholangiocarcinoma can be differentiated from other liver tumors in the majority of patients. A well-defined mass centered on the central hepatic ducts, which shows inhomogeneous and progressive enhancement following gadolinium, prominent intrahepatic duct dilatation distal to the tumor, atrophy of the lobe involved by tumor, and portal venous occlusion, is strongly suggestive of the diagnosis of cholangiocarcinoma.
Extrahepatic cholangiocarcinoma usually presents as circumferential thickening and delayed enhancement of the bile duct wall. It can be difficult to differentiate from pancreatic head cancer.
Preoperative imaging with MRI/MRA/MRCP is an accurate non-invasive method for staging cholangiocarcinoma, and determining resectability. It provides information regarding tumor size, extent of bile duct involvement by tumor, vascular patency, extrahepatic extension, nodal or distant metastases, and the presence of lobar atrophy. MRCP is better for demonstrating bile ducts distal to the stricture, although with ERCP, therapeutic intervention such as stent placement and biopsy can be performed.
We recommend the following imaging protocol for cholangiocarcinoma:
T1W dual-echo SGRE (breath-hold). T1W spin-echo can also be used.
T2W fast spin-echo (FSE) with fat suppression and respiratory triggering. Alternative sequences can be used such as the breath-hold T2W fast recovery fast spin echo sequence (FRFSE), and the breath-hold short tau inversion recovery (Turbo STIR). The single shot fast spin echo (SSFSE or HASTE) sequence should not be used as the only T2W sequence as solid tumors may not be depicted on this sequence.
Dynamic gadolinium-enhanced 3D SGRE, acquired in the arterial-dominant, portal venous (sinusoidal), interstitial (2 min), and delayed (15 min of enhancement). The arterial-dominant phase is used to assess for patency of hepatic arteries; portal-venous and interstitial phases to assess patency of portal and hepatic veins, and evaluate liver parenchyma, extrahepatic disease, lymph node involvement, and location and extent of primary tumor; 15 min delayed phase for assessment of tumor extent, and metastatic disease in the liver.
MRCP: 3D FRFSE respiratory triggered MRCP, or standard SSFSE (HASTE) multiplanar thin slice and thick slab MRCP.
References
- 1.Buetow P, Midkiff R. Primary malignant neoplasms in the adult. MRI Clin N Am. 1997;5(2):289–318. [PubMed] [Google Scholar]
- 2.Vilgrain V, Van Beers BE, Flejou JF, et al. Intrahepatic cholangiocarcinoma: MRI and pathologic correlation in 14 patients. J Comput Assist Tomogr. 1997;21(1):59–65. doi: 10.1097/00004728-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Hamrick-Turner J, Abbitt PL, Ros PR. Intrahepatic cholangiocarcinoma: MR appearance. AJR Am J Roentgenol. 1992;158(1):77–9. doi: 10.2214/ajr.158.1.1309221. [DOI] [PubMed] [Google Scholar]
- 4.Szklaruk J, Tamm E, Charnsangavej C. Preoperative imaging of biliary tract cancers. Surg Oncol Clin N Am. 2002;11(4):865–76. doi: 10.1016/s1055-3207(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 5.Craig JR, Peters RL, Edmonson HA. Atlas of Tumor Pathology. 2nd series, fascicle 26. Washington, DC: Armed Forces Institute of Pathology; 1988. Tumors of the liver and intrahepatic bile ducts; pp. 16B–43. [Google Scholar]
- 6.Blumgart LH, Fong Y, Jarnagin W. American Cancer Society Atlas of Clinical Oncology Series. Hamilton, Ontario: BC Decker; 2001. Hepatobiliary cancer; pp. 193–209. [Google Scholar]
- 7.Ros PR, Buck JL, Goodman ZD, Ros AM, Olmsted WW. Intrahepatic cholangiocarcinoma: radiologic pathologic correlation. Radiology. 1988;167:689–93. doi: 10.1148/radiology.167.3.2834769. [DOI] [PubMed] [Google Scholar]
- 8.Manfredi R, Masselli G, Maresca G, Brizi MG, Vecchioli A, Marano P. MR imaging and MRCP of hilar cholangiocarcinoma. Abdom Imaging. 2003;28:319–25. doi: 10.1007/s00261-002-0047-x. [DOI] [PubMed] [Google Scholar]
- 9.Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19:156–76. doi: 10.1002/1098-2388(200009)19:2<156::aid-ssu8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Pavone P. MR cholangiopancreatography in malignant biliary obstruction. Semin Ultrasound CT MRI. 1999;20(5):317–23. doi: 10.1016/s0887-2171(99)90063-x. [DOI] [PubMed] [Google Scholar]
- 11.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–19. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahrendt SA, Nakeeb A, Pitt HA. Cholangiocarcinoma. Clin Liver Dis. 2001;5(1):191–218. doi: 10.1016/s1089-3261(05)70161-6. [DOI] [PubMed] [Google Scholar]
- 13.Choi B. MRI of clonorchiasis and cholangiocarcinoma. J Magn Reson Imaging. 1998;8(2):359–66. doi: 10.1002/jmri.1880080215. [DOI] [PubMed] [Google Scholar]
- 14.Khan SA, Davidson BR, Goldin R, et al. British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy AD. Malignant liver tumors. Clin Liver Dis. 2002;6(1):147–64. doi: 10.1016/s1089-3261(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 16.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg. 1998;228(3):411–20. doi: 10.1097/00000658-199809000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrendt SA, Pitt HA, Nakeeb A, et al. Diagnosis and management of cholangiocarcinoma in primary sclerosing cholangitis. J Gastrointest Surg. 1999;3(4):357–67. doi: 10.1016/s1091-255x(99)80051-1. [DOI] [PubMed] [Google Scholar]
- 18.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–73. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341(18):1368–78. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 20.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241–56. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;12(4):288–91. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 22.Lee WJ, Lim HK, Jang KM, et al. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics. 2001;21:S97–S116. doi: 10.1148/radiographics.21.suppl_1.g01oc12s97. [DOI] [PubMed] [Google Scholar]
- 23.Nakanuma Y, Harada K, Ishikawa A, Zen Y, Sasaki M. Anatomic and molecular pathology of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10(4):265–81. doi: 10.1007/s00534-002-0729-3. [DOI] [PubMed] [Google Scholar]
- 24.Rubin E, Farber J. Pathology. 2nd edn. Philadelphia, PA: J.B. Lippincott; 1994. [Google Scholar]
- 25.Maetani Y, Itoh K, Watanabe C, et al. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. AJR Am J Roentgenol. 2001;176(6):1499–507. doi: 10.2214/ajr.176.6.1761499. [DOI] [PubMed] [Google Scholar]
- 26.Kehagias D, Metafa A, Hatziioannou A, et al. Comparison of CT, MRI and CT during arterial portography in the detection of malignant hepatic lesions. Hepatogastroenterology. 2000;47(35):1399–403. [PubMed] [Google Scholar]
- 27.Soyer P, Bluemke DA, Reichle R, et al. Imaging of intrahepatic cholangiocarcinoma: 2. Hilar cholangiocarcinoma. AJR Am J Roentgenol. 1995;165(6):1433–6. doi: 10.2214/ajr.165.6.7484580. [DOI] [PubMed] [Google Scholar]
- 28.Hultcrantz R, Olsson R, Danielsson A, et al. A 3-year prospective study on serum tumor markers used for detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 1999;30(4):669–73. doi: 10.1016/s0168-8278(99)80198-6. [DOI] [PubMed] [Google Scholar]
- 29.Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108(3):865–9. doi: 10.1016/0016-5085(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 30.Baer H, Stain S, Dennison A, et al. Improvements in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg. 1992;217:20–7. doi: 10.1097/00000658-199301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–8. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langer JC, Langer B, Taylor BR, et al. Carcinoma of the extrahepatic bile ducts: results of an aggressive surgical approach. Surgery. 1985;98:752–9. [PubMed] [Google Scholar]
- 33.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170–8. [PubMed] [Google Scholar]
- 34.Lee SS, Kim MH, Lee SK, et al. MR cholangiography versus cholangioscopy for evaluation of longitudinal extension of hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56(1):25–32. doi: 10.1067/mge.2002.125363. [DOI] [PubMed] [Google Scholar]
- 35.Soyer P, Bluemke DA, Reichle R, et al. Imaging of intrahepatic cholangiocarcinoma: 1. Peripheral cholangiocarcinoma. AJR Am J Roentgenol. 1995;165:1427–31. doi: 10.2214/ajr.165.6.7484579. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr. 1999;23(5):670–7. doi: 10.1097/00004728-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Murakami T, Nakamura H, Tsuda K, et al. Contrast-enhanced MR imaging of intrahepatic cholangiocarcinoma: pathologic correlation study. J Magn Reson Imaging. 1995;5(2):165–70. doi: 10.1002/jmri.1880050210. [DOI] [PubMed] [Google Scholar]
- 38.Low RN. MR imaging of the liver using gadolinium chelates. Magn Reson Imaging Clin N Am. 2001;9(4):717–43. [PubMed] [Google Scholar]
- 39.Worawattanakul S, Semelka RC, Noone TC, Calvo BF, Kelekis NL, Woosley JT. Cholangiocarcinoma: spectrum of appearances on MR images using current techniques. Magn Reson Imaging. 1998;16(9):993–1003. doi: 10.1016/s0730-725x(98)00135-0. [DOI] [PubMed] [Google Scholar]
- 40.Greco A, Stipa F, Huguet C, Gavelli A, Chieco PA, McNamara MT. Early MR follow-up of partial hepatectomy. J Comput Assist Tomogr. 1993;17(2):277–82. doi: 10.1097/00004728-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Soyer P, Bluemke DA, Sibert A, Laissy JP. MR imaging of intrahepatic cholangiocarcinoma. Abdom Imaging. 1995;20(2):126–30. doi: 10.1007/BF00201519. [DOI] [PubMed] [Google Scholar]
- 42.Dooms GC, Kerlan RK Jr, Hricak H, Wall SD, Margulis AR. Cholangiocarcinoma: imaging by MR. Radiology. 1986;159:89–94. doi: 10.1148/radiology.159.1.3006122. [DOI] [PubMed] [Google Scholar]
- 43.Guthrie JA, Ward J, Robinson PJ. Hilar cholangiocarcinomas: T2-weighted spin-echo and gadolinium-enhanced FLASH MR imaging. Radiology. 1996;201(2):347–51. doi: 10.1148/radiology.201.2.8888221. [DOI] [PubMed] [Google Scholar]
- 44.Awaya H. Differential diagnosis of hepatic tumors with delayed enhancement at gadolinium-enhanced MRI: a pictorial essay. Clin Imaging. 1998;22:180–7. doi: 10.1016/s0899-7071(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 45.Gabata T, Matsui O, Kadoya M, et al. Delayed MR imaging of the liver: correlation of delayed enhancement of hepatic tumors and pathologic appearance. Abdom Imaging. 1998;23(3):309–13. doi: 10.1007/s002619900347. [DOI] [PubMed] [Google Scholar]
- 46.Peterson MS, Murakami T, Baron RL. MR imaging patterns of gadolinium retention within liver neoplasms. Abdom Imaging. 1998;23(6):592–9. doi: 10.1007/s002619900410. [DOI] [PubMed] [Google Scholar]
- 47.Fan ZM, Yamashita Y, Harada M, et al. Intrahepatic cholangiocarcinoma: spin-echo and contrast-enhanced dynamic MR imaging. AJR Am J Roentgenol. 1993;161(2):313–7. doi: 10.2214/ajr.161.2.8392787. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita Y. Parenchymal changes of the liver in cholangiocarcinoma: CT evaluation. Gastrointest Radiol. 1992;17:161–6. doi: 10.1007/BF01888536. [DOI] [PubMed] [Google Scholar]
- 49.Semelka R. Abdominal-Pelvic MRI. New York: Wiley-Liss; 2002. [Google Scholar]
- 50.Yoshimitsu K, Honda H, Kaneko K, et al. MR signal intensity changes in hepatic parenchyma with ductal dilation caused by intrahepatic cholangiocarcinoma. J Magn Reson Imaging. 1997;7(1):136–41. doi: 10.1002/jmri.1880070119. [DOI] [PubMed] [Google Scholar]
- 51.Low RN, Sigeti JS. MR imaging of peritoneal disease: comparison of contrast-enhanced fast multiplanar spoiled gradient-recalled and spin-echo imaging. AJR Am J Roentgenol. 1994;163(5):1131–40. doi: 10.2214/ajr.163.5.7976889. [DOI] [PubMed] [Google Scholar]
- 52.Low RN, Barone RM, Lacey C, Sigeti JS, Alzate GD, Sebrechts CP. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997;204(2):513–20. doi: 10.1148/radiology.204.2.9240546. [DOI] [PubMed] [Google Scholar]
- 53.Itai Y, Ohtomo K, Kokubo T, et al. CT of hepatic masses: significance of prolonged and delayed enhancement. AJR Am J Roentgenol. 1986;146:729–33. doi: 10.2214/ajr.146.4.729. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez M, Redyanly RD. Primary hepatic malignant neoplasms. Radiol Clin N Am. 1998;36(2):333–48. doi: 10.1016/s0033-8389(05)70026-9. [DOI] [PubMed] [Google Scholar]
- 55.Lee MG, Park KB, Shin YM, et al. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg. 2003;27(3):278–83. doi: 10.1007/s00268-002-6701-1. [DOI] [PubMed] [Google Scholar]
- 56.Hann LE, Schwartz LH, Panicek DM, Bach AM, Fong Y, Blumgart LH. Tumor involvement in hepatic veins: comparison of MR imaging and US for preoperative assessment. Radiology. 1998;206(3):651–6. doi: 10.1148/radiology.206.3.9494482. [DOI] [PubMed] [Google Scholar]
- 57.Yeh TS, Jan YY, Tseng JH, et al. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000;95(2):432–40. doi: 10.1111/j.1572-0241.2000.01763.x. [DOI] [PubMed] [Google Scholar]
- 58.Reinhold C, Bret PM. Current status of MR cholangiopancreatography. AJR Am J Roentgenol. 1996;166:1285–95. doi: 10.2214/ajr.166.6.8633434. [DOI] [PubMed] [Google Scholar]
- 59.Fulcher AS, Turner MA. HASTE MR cholangiography in the evaluation of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1997;169(6):1501–5. doi: 10.2214/ajr.169.6.9393153. [DOI] [PubMed] [Google Scholar]
- 60.Thng C, Tan A, Chung Y, Chow P, Ooi L. Clinical applications of MR cholangiopancreatography. Ann Acad Med Singapore. 2003;32(4):536–41. [PubMed] [Google Scholar]
- 61.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 6th edn. New York: International Union Against Cancer/Wiley-Liss; 2002. [Google Scholar]