Abstract
Metastatic cervical lymphadenopathy is a common problem in head and neck oncology. The appropriate management of the cervical lymph nodes requires a good understanding of the incidence, patterns, and prognostic implications of nodal metastasis. This paper correlates the anatomical and the simplified level classification systems of cervical lymph nodes, examines the clinical significance of nodal metastasis, and evaluates the criteria for nodal metastasis.
Keywords: Cervical nodes, metastatic disease, nodal extracapsular spread, nodal necrosis
Introduction
Most head and neck tumours spread to the neck nodes as part of their natural history. Depending on the primary site, up to 80% of patients with upper aerodigestive mucosal malignancy will have cervical nodal metastasis at presentation. The occurrence of nodal metastasis has a profound effect on the management and prognosis of these patients. Cervical nodal metastases are therefore a very common clinical problem. Radiologists should be familiar with the simplified level classification system currently used by both head and neck surgeons and pathologists who use the same system when they report on the involvement of cervical nodes in radical neck dissection specimens.
This paper will review the classical anatomy of cervical nodes and correlate the traditional nomenclature with the simplified level classification system. The criteria for the diagnosis of metastatic nodes will be described and their significance in patient management evaluated.
Cervical nodes classification system
Rouviere classified cervical nodes into a collar of nodes surrounding the upper aerodigestive tract (submental, facial, submandibular, parotid, mastoid, occipital and retropharyngeal) and two groups along the long axis of the neck (anterior cervical and postero-lateral cervical groups) [1]. Surgeons, however, make use of the simplified level system advocated by Shah et al. at the Memorial Sloan-Kettering Cancer Center, New York [2, 3]. Table 1 correlates the simplified level system and the corresponding anatomical location of cervical nodes.
Table 1.
Simplified numerical classification system
| Level | Location |
|---|---|
| IA | Submental lymph nodes |
| IB | Submandibular lymph nodes |
| II | Internal jugular (deep cervical) chain from the base of the skull to the inferior border of the hyoid bone |
| III | Internal jugular (deep cervical) chain from the hyoid bone to the inferior border of the cricoid arch |
| IV | Internal jugular (deep cervical) chain between the inferior border of the cricoid arch and the supraclavicular fossa |
| V | Posterior triangle or spinal accessory nodes |
| VI | Central compartment nodes from the hyoid bone to the suprasternal notch |
| VII | Nodes inferior to the suprasternal notch in the upper mediastinum |
In practical terms, the Group IA (submental) nodes are located in the submental space, between the anterior bellies of the digastric muscles (Fig. 1). Group IB (submandibular) nodes are found in the submandibular space, around the submandibular gland (Fig. 2). Groups II, III and IV are internal jugular (deep cervical) nodes and they are divided into these three groups by two landmarks: the hyoid bone and the inferior border of the cricoid cartilage. Hence, Group II nodes are located above the hyoid cartilage (Fig. 3), Group III nodes are found between the hyoid bone and cricoid cartilage (Fig. 4), and Group IV nodes are located below the cricoid cartilage (Fig. 5). Group V nodes are found in the posterior triangle (Fig. 6). Group V nodes can be identified on axial images posterior to the posterior margin of the sternocleidomastoid muscle. Group VI nodes are anteriorly located: between the hyoid bone superiorly, the suprasternal notch inferiorly and between the carotid sheaths laterally (Fig. 7).
Figure 1.
Contrast-enhanced CT shows multiple enlarged Group IA (submental) nodes. Note the presence of nodal necrosis (arrow).
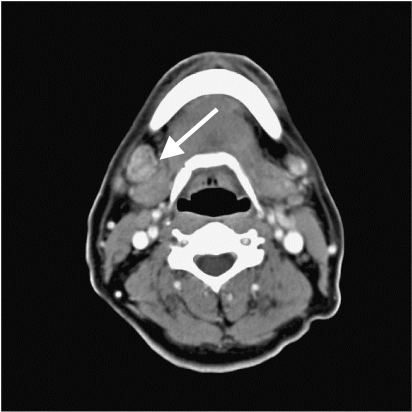
Figure 2.
Contrast-enhanced CT shows an enlarged right Group IB (submandibular) lymph node (arrow).
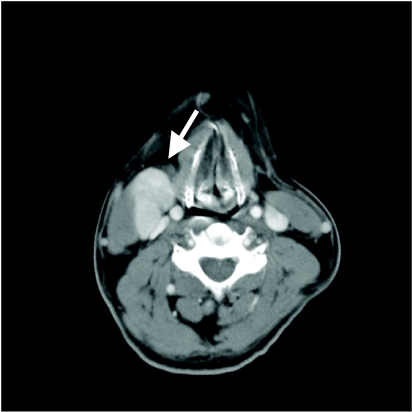
Figure 3.
Contrast-enhanced CT shows left level II lymphadenopathy (white arrow). Level II nodes are internal jugular nodes above the level of the hyoid bone. Note the carcinoma (black arrow) in the tongue base.
Figure 4.
Contrast-enhanced CT shows a right enlarged level III node (arrow) with intense enhancement. Level III nodes are internal jugular nodes located between the hyoid bone and cricoid cartilage landmarks.
Figure 5.
Contrast-enhanced CT shows a right enlarged level IV lymph node (white arrow) which mimics a vessel. Note the previous resected right thyroid lobe (because of papillary carcinoma) and the jugular vein (black arrow). Level IV nodes are internal jugular nodes located below the cricoid cartilage.
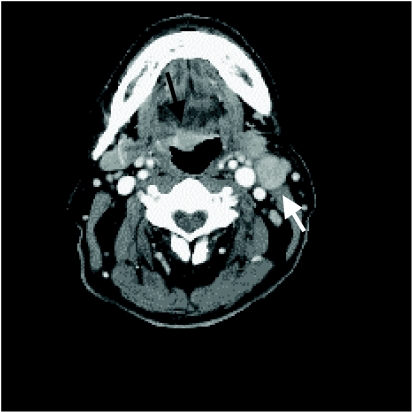
Figure 6.
Contrast-enhanced CT shows an enlarged level V (arrow). Level V nodes are located in the posterior triangle. On CT they are seen posterior to the posterior margin of the sternocleidomastoid muscle.
Figure 7.
Contrast-enhanced CT shows enlargement of level VI nodes (arrow). Level VI nodes (located anteriorly and centrally) are found adjacent to the thyroid gland, thyroid cartilage, larynx and oesophagus.
The facial, parotid, mastoid, occipital and retropharyngeal are not included in the simplified level classification system. This is because the level system was first introduced within the framework of neck dissection and these nodes are not normally included in the surgical procedure. If these nodes are enlarged, they are given their anatomical names. It should be noted that the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) does not accept the designation of the upper anterior mediastinal nodes as Group VII nodes used by the American Joint Committee on Cancer (AJCC). Amongst other reasons, it was felt that the nodes included in Group VII are in fact anatomically mediastinal nodes and as such should not be included in the classification of neck nodes.
Clinical significance of metastatic cervical lymphadenopathy
Nodal metastasis is the single most important prognostic factor in squamous cell carcinoma (SCC) of the head and neck. In general, it decreases the overall survival by half [4]. Extracapsular spread worsens the prognosis by another half [5]. The level of nodal metastasis, and the number and size of nodes are also significant and these factors correlate with distant metastasis [4, 6, 7].
Imaging cervical nodes
Nodes larger than 10 mm are conventionally considered abnormal. However, 20% of nodes that exceed 10 mm harbour no metastatic deposits and histologically show only hyperplasia. On the other hand, 23% of nodes that show extracapsular spread measure less than 10 mm [8]. The presence of nodal necrosis, irrespective of size, indicates metastatic involvement. Although this sign is highly specific for metastatic disease it is of limited usefulness in clinical practice. This is because most nodes with nodal necrosis are larger than 10 mm. These nodes are already by size criterion considered as nodes affected by metastasis (Fig. 1). In general, the frequency of nodal necrosis increases with nodal size. The detection of nodal necrosis is therefore most useful if the necrotic nodes are less than 10 mm and there are no other abnormal nodes.
Nodal necrosis may be confused in two conditions. Firstly, fat deposition may produce a low attenuation focus in the suspected node on computed tomography (CT). Density measurements are of limited value in small lesions because of partial volume averaging. The location of the low attenuation focus is of help as necrosis is generally situated centrally while fat is usually deposited around the hilum. Secondly, suppurative nodes frequently show central areas of low attenuation indicating the formation of pus. These nodes usually have irregular and ill-defined margins indicating the presence of inflammation. Suppurative lymphadenitis is usually evident clinically and radiologically. The presence of cellulitis helps to separate metastasis from inflammation.
Extracapsular spread is common with approximately 60% of all metastatic nodes showing extracapsular spread. Extracapsular spread is diagnosed when the nodes appear matted or the nodal outline appears streaky. Imaging is not very sensitive and approximately 45% of all histologically verified nodes with extracapsular spread are not seen on CT [8]. It should also be noted that 50% of nodes harbouring malignant cells measure less than 5 mm and 25% of nodes with extracapsular spread are less than 10 mm.
The role of imaging
The main roles of imaging are (1) to confirm the N0 status of the neck, (2) to document lymphadenopathy contralateral to clinically palpable disease, (3) to assess the regional extent of disease especially in relation to neurovascular structures, and (4) nodal surveillance for follow-up.
One of the greatest challenges in the management of nodal metastasis is the issue of clinical N0 disease. In other words, what should be done in patients who are likely to harbour occult metastasis? The occult metastasis rates are very high in some primary lesions (oral cavity 41%; oropharynx 36%; hypopharynx 36%; and supraglottic tumours 29%) [9]. If there is more than a 20% chance of occult metastasis, most surgeons will perform elective neck dissection. In these patients, even if imaging shows no lymphadenopathy, neck dissection will be done when the primary lesion is excised.
There are other primary sites with low occult metastatic disease rates. Malignancies originating from the parotid gland, maxillary sinus or glottic carcinomas have typically less than a 5% chance of occult metastasis. In these patients, if the lymph nodes are not radiologically enlarged, the patients will be spared neck dissection.
Extracapsular spread can invade the carotid sheath rendering the patient a non-surgical candidate. In spite of tremendous advances in surgical and anaesthetic techniques, most surgeons will not operate when the carotid artery is involved because of the high mortality and morbidity rates. The detection of carotid artery involvement is therefore of crucial importance. In this respect, the accuracy of imaging is disappointing. It is commonly known that a large area of contact between a nodal mass and the carotid artery documented on imaging studies proves negative for invasion during surgery. On the other hand, some patients with small areas of contact on imaging were found to have adventitial invasion. Yousem et al. reported that the presence of more than 270^ of circumferential involvement of the carotid artery was highly suggestive of unresectability [10].
One of the greatest contributions of imaging is in the follow-up of patients. Following surgery or radiation therapy or both, the indurated soft tissues often make neck palpation difficult. Enlarged nodes are much more easily identified on imaging. This is especially so when serial follow-up studies detect nodal enlargement before it becomes palpable (Fig. 8).
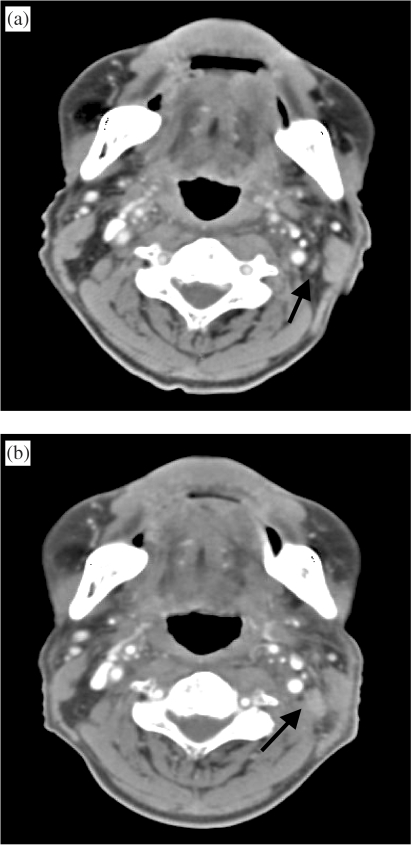
Figure 8.
(a) Contrast-enhanced CT shows no significant cervical lymphadenopathy following neck irradiation for nasopharyngeal cancer. Note the innocuous looking lymph node (arrow). (b) Follow-up (1 year later) shows definite enlargement of cervical node (arrow). The node was not palpable because of its size, its location beneath the sternocleidomastoid muscle and the irradiation-induced neck induration.
In summary, radiologists should be familiar with the anatomical distribution of cervical nodes and their relationship with the simplified level classification system used by their surgical colleagues. Imaging reports should therefore document lymphadenopathy using a common classification system to facilitate communication. Radiologists should also be familiar with prognostic implications of nodal metastasis, the limitations of imaging and their role in the detection of lymphadenopathy.
References
- 1.Rouviere H. Anatomy of the Human Lymphatic System. Ann Arbor, MI: Edward Brothers; 1938. [Google Scholar]
- 2.Shah JP, Strong E, Spiro RH, Vikram B. Surgical grand rounds: neck dissection: current status and future possibilities. Clin Bull. 1981;11:25–33. [PubMed] [Google Scholar]
- 3.Som PM, Curtin HD, Mancuso AA. An imaging-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classification. Arch Otolaryngol Head Neck Surg. 1999;125:388–96. doi: 10.1001/archotol.125.4.388. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien CJ, Smith JW, Soong SJ, Urist MM, Maddox WA. Neck dissections with and without radiotherapy—prognostic factors, patterns of recurrence and survival. Am J Surg. 1986;152:456–63. doi: 10.1016/0002-9610(86)90324-7. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JT, Barnes EL, Myers EN. The extracapsular spread of tumors in cervical nodal metastasis. Arch Otolaryngol Head Neck Surg. 1981;107:725–8. doi: 10.1001/archotol.1981.00790480001001. [DOI] [PubMed] [Google Scholar]
- 6.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastasis in head and neck carcinoma. Cancer. 1993;71:452–6. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Teo PML, Leung SF, Yu P, Tsao SY, Foo W, Shiu W. A comparison of Ho’s, International Union Against Cancer, and American Joint Committee stage classifications for nasopharyngeal carcinoma. Cancer. 1991;167:434–9. doi: 10.1002/1097-0142(19910115)67:2<434::aid-cncr2820670219>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Som PM. Detection of metastasis in cervical nodes: CT and MR criteria and differential diagnosis. Am J Roentgenol. 1992;158:961–9. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 9.Medina JE, Houck JR, O’Malley BB. Management of cervical lymph nodes in SCC of head and neck. In: Harrison LB, Sessions RB, Hong WK, editors. Head and Neck Cancer. Philadelphia, PA: Lippincott-Raven; 1999. pp. 353–77. [Google Scholar]
- 10.Yousem DM, Hatabu H, Hurst RW, et al. Carotid artery invasion by head and neck masses: prediction by MR imaging. Radiology. 1995;195:715–20. doi: 10.1148/radiology.195.3.7754000. [DOI] [PubMed] [Google Scholar]