Abstract
Embolisation has become an accepted modality of cancer treatment in patients with a variety of clinical scenarios. It is commonly used in clinical practice in the treatment of hepatocellular carcinoma, hepatic metastases from colorectal cancer and neuroendocrine tumours, and renal cell carcinoma. This review summarizes the current evidence for the efficacy of embolotherapy in these clinical settings, together with the associated complications.
Keywords: Embolisation, chemoembolisation, hepatocellular carcinoma, cancer
Introduction
Embolisation is the deliberate occlusion of a vessel, and is performed to reduce or stop the flow of blood into that vessel. Usually the aim of embolisation in the treatment of cancer is to produce ischaemia within the tumour, resulting in tumour necrosis. This effect may be potentiated by the addition of a chemotherapeutic agent to the embolic material, which is termed chemoembolisation. Embolisation may also be performed to arrest life-threatening haemorrhage due to a tumour.
The choice of embolisation agent depends largely upon the indication. Metal coils will occlude vessels and fibres attached to them cause thrombosis, similar to the effect of surgical ligation. Particulate agents such as polyvinyl alcohol (PVA) particles flow downstream until they become blocked in the corresponding-sized vessel; sizes ranging from 100 to 1000 μm are normally used. Soluble gelatine sponge may be cut into pledgets for occluding larger vessels or made into a slurry; it is said to be a temporary agent with vessel recanalisation occurring in a few weeks. Alcohol is a liquid agent that causes cell death by denaturation. Lipiodol is iodised poppy seed oil. It is used in conjunction with chemotherapy in transcatheter arterial chemoembolisation (TACE), since it acts as an embolic agent, probably at the level of small venules and is selectively taken up by some tumours, such as hepatocellular carcinomas (HCC) and renal cell carcinomas.
In clinical practice the common uses of embolisation are as follows: TACE of HCC, where curative resection or transplantation is not possible; TACE of colorectal hepatic metastases, again where resection is not possible; TACE of hepatic neuroendocrine metastases with the goal of reducing systemic symptoms of the carcinoid syndrome; embolisation of large vascular renal cell cancers to render nephrectomy safer; and embolisation of unresectable renal cell carcinoma to prevent haematuria or treat local symptoms such as pain. We aim to review the evidence supporting the use of embolotherapy in these clinical settings.
HCC
HCC is one of the commonest malignancies world-wide, largely due to the high prevalence of hepatitis B infection in the developing world. Despite being a rare cancer in the western hemisphere there is evidence that its incidence is increasing [1, 2], which may be attributed to the rising prevalence of hepatitis B and C in the western hemisphere [1, 3]. Untreated, HCC has a poor prognosis, with surgical resection or liver transplantation offering the most effective therapy and the only possibility of a cure [4]. However, few patients are candidates for these treatments and so percutaneous radiological interventions, including TACE, are becoming the mainstay of treatment for many with this disease.
Technique
The normal liver has a dual blood supply, and derives approximately 75% of its supply from the portal vein. In contrast, primary and secondary liver tumours derive around 90% of their blood supply from the hepatic artery. In view of this, hepatic artery embolisation appears to have the ability to preferentially target tumour while sparing liver parenchyma. The goal of embolisation is to induce tumour necrosis by rendering the tumour ischaemic. The addition of chemotherapy to the embolic material results in high local concentrations of chemotherapeutic agent, with little reaching the systemic circulation due to the vessel occlusion. Furthermore, the local ischaemia is thought to disrupt cell membrane transport, preventing removal of the chemotherapeutic agent from the cell. Lipiodol has been shown to be taken up by tumour cells, and so is commonly used as a carrier for the chemotherapeutic agent, as it also functions as an embolic agent (Figs 1 and 2).
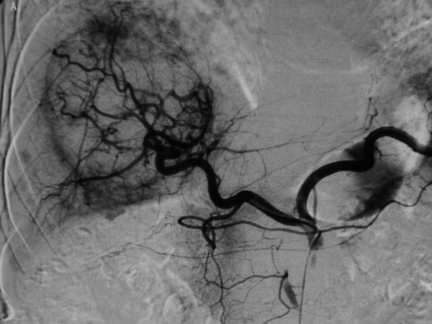
Figure 1.
Coeliac angiography demonstrates a large hepatocellular carcinoma supplied by hepatic artery. Note the small tumour blushes adjacent to the main tumour.
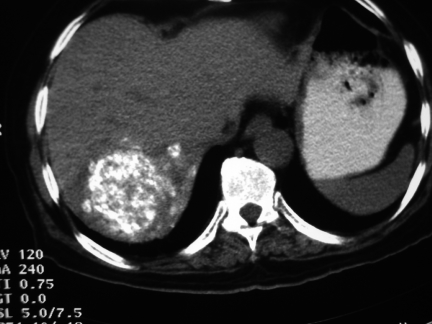
Figure 2.
CT following chemoembolisation shows lipiodol uptake in the tumour and confirms satellite tumours.
The procedure involves selectively catheterising the hepatic artery; in some cases, for example where there is only a single tumour present, superselective catheterisation of the artery feeding the tumour may be performed. For multiple or ill-defined tumours less selective catheterisation is necessary to embolise the entire tumour mass.
In practice there is a wide variation in the protocols of embolisation described. Some studies have used transcatheter arterial embolisation (TAE), without chemotherapy added, utilising ischaemia to induce tumour necrosis. Hepatic arterial infusion of chemotherapy without embolisation has also been used. Many differing protocols of TACE have been described, with a variety of embolic materials used, including lipiodol, and with differing chemotherapeutic agents added. Some authors have found advantage with platinum-based regimens over regimens with anthracyclines such as doxorubicin or epirubicin [5]. However, there are few papers prospectively comparing regimens, and while some have favoured one regimen over another there is no clear consensus. Most regimens use an anthracycline or platinum agent in combination with lipiodol, often with additional embolic agent such as soluble gelatine sponge.
Efficacy
A large number of studies have been published on the use of TACE in the treatment of HCC, and until recently no clear effect on survival had been demonstrated. This is largely because of wide differences between trials in patient selection criteria, TACE protocol and measures of response to treatment used. The effect of patient selection is important on influencing survival; poor prognostic indicators prior to therapy in one study were shown to be α-fetoprotein >400 U/l, tumour size >50% of liver volume and Child–Pugh class C [6] and in another study [7] extra-hepatic metastases, tumour extension, ascites and icterus were adverse prognostic factors. Hatanaka et al. [7] also compared four differing protocols of TACE, with tumour characteristics determining the protocol used. Two protocols that included soluble gelatine sponge showed a superior survival rate than those that did not, and the inclusion of a chemotherapeutic agent produced no survival benefit. Overall, however, the prognostic factors described above were more important than treatment protocol for prognosis; this has also been demonstrated in a study by Ueno et al. [8].
Measures of response to treatment used have included imaging decrease of size of tumour, degree of tumour necrosis, lipiodol uptake by tumour, decrease in α-fetoprotein, symptomatic improvement and survival. The ability of TACE to cause tumour necrosis and reduce tumour volume is well established, with a reported range of extent of necrosis of 60–100% [3]. However, the effect of TACE on survival has proved harder to demonstrate. Numerous case-control studies have shown a survival benefit with TACE, with 1-year survival rates as high as 100% for patients with small tumours and 59% for patients with large tumours. Level 1 or 2 evidence for an effect on survival has until recently been lacking. Several randomised controlled trials (RCTs) have been published comparing TACE with symptomatic treatment with variable results. Three of these failed to demonstrate a significant survival benefit in patients [9–11], despite all three showing an objective tumour response to treatment. Other randomised controlled trials have shown increased survival following treatment with TACE. Bayraktar et al. [12] compared TACE with systemic chemotherapy and no treatment in patients with advanced HCC; mean survival in the TACE group was 13 months, compared with 7.2 months in the chemotherapy group and 6.9 months in the untreated group. Lo et al. [13] randomised 80 patients with unresectable HCC to receive either TACE or symptomatic treatment; 1-year, 2-year and 3-year survival rates were 57, 31 and 26% in the TACE group and 32, 11 and 3% in the symptomatic treatment group. Finally the study by Llovet et al. [14] was stopped after interim analysis showed a survival benefit for TACE over conservative therapy. This trial compared conservative therapy, TAE and TACE in 112 patients. Survival rates at 1 and 2 years were 63 and 27% for controls, 75 and 50% for TAE and 82 and 63% for TACE. Two recent meta-analyses of randomised controlled trials comparing TACE with conservative therapy have confirmed the increased survival associated with TACE therapy [15, 16]. Camma et al. analysed the results of five RCTs of chemoembolisation against conservative management. They also analysed a further 13 trials comparing different procedures, i.e. chemoembolisation versus intra-arterial chemotherapy alone or embolisation alone. They found that there is sufficient evidence to show that chemoembolisation reduces 2-year mortality in patients with HCC, and that chemoembolisation was not more effective than embolisation alone, suggesting that the currently used chemotherapeutic agents are not optimally effective.
On the basis of the evidence reviewed, the authors did not recommend chemoembolisation for all patients with HCC. Patients with poor Child–Pugh status, evidence of hepatic decompensation or portal vein thrombosis are often excluded, but subgroup analysis of these groups was not possible because of missing data from several trials. Similarly, no conclusions could be drawn regarding the effectiveness of multiple treatments versus single treatment, or the effect of superselective embolisation of tumour [15].
TACE has also been used in conjunction with other modalities of treatment. TACE may be performed pre-operatively for resectable tumours in an attempt to improve disease-free survival. Zhang et al. have published a large series comparing patients who received TACE before hepatectomy with patients who did not [17]. Five-year disease-free survival was 51% in patients who received TACE twice, 35.5% in patients who received TACE once and 21.4% in patients who did not receive treatment with TACE pre-operatively. TACE has also been used to delay tumour progression in patients awaiting liver transplantation, and so reduce the proportion of patients who become ineligible for transplantation before a donor liver becomes available [18]. For patients with small tumours who are not candidates for hepatectomy or transplantation other locoregional treatments that have proved effective include percutaneous ethanol injection (PEI) and various thermal ablative techniques. PEI results in survival rates similar to surgery [19] when used to treat small tumours; for larger tumours there is evidence that the combination of TACE with PEI is effective [20–22]. Recently, thermal ablative techniques such as radiofrequency (RF) ablation have been shown to be as effective or superior to PEI [23]. The addition of TACE may be used in conjunction with thermal ablative techniques for tumours considered too large for thermal ablation alone [24].
Complications
‘Post-embolisation syndrome’ is a common complication of TACE, reported in as many as 86% of patients [9], and consists of abdominal pain, nausea and vomiting, and pyrexia for 48–72 h after the procedure, and is treated prophylactically and supportively with opiate analgesia and anti-emetics. Acute liver failure becomes increasingly likely with larger tumours, infiltrating tumours, increasing number of tumours, severity of underlying liver dysfunction and presence of portal vein thrombosis, and it is the degree of risk of precipitating acute liver failure that limits the use of TACE. Biliary sepsis, gallbladder infarction and hepatic abscess (Figs 3 and 4) are also possible complications, and are predisposed to by local biliary obstruction due to tumour. Acute liver decompensation has been reported in between 20 and 50% of patients, although in most cases this was reversible [9, 25]. Mortality is low, ranging from 0 to 4%, with causes of death reported as due to liver failure [10, 11], bleeding from oesophageal varices [11] and hepatic and splenic abscesses [25].
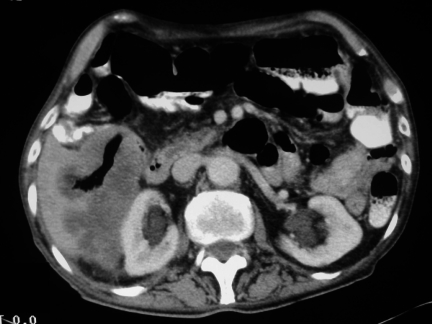
Figure 3.
CT of the liver after embolisation demonstates abscess formation with fluid and air seen within the liver substance.
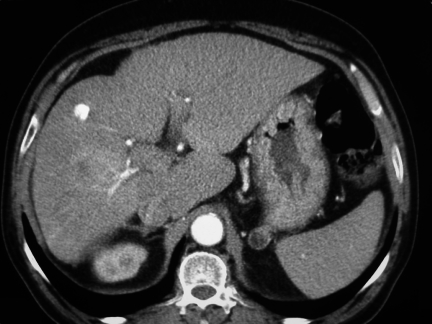
Figure 4.
CT after TACE demonstrates lipiodol uptake in treated area but also a new area of increased enhancement in the right lobe indicative of progressive disease.
Hepatic metastases
The underlying rationale for the use of TACE in managing hepatic metastases is the same as that for HCC; that normal liver receives 75% of its blood supply from the portal vein whereas tumour derives 90% of its supply from the hepatic artery, and that the combination of relatively high local concentration of chemotherapeutic agent and ischaemia due to loss of blood supply will result in tumour necrosis. Neuroendocrine metastases are hypervascular tumours that may be expected to respond particularly well to TACE. The technique is as for HCC.
Colorectal hepatic metastases
For patients with colorectal carcinoma, it is usually the presence of hepatic metastases that limits survival. For carefully selected patients surgical resection of the involved portion of liver can offer some hope of long-term survival, with 5-year survival following resection for metastases of 35–40% [26]. Selective TAE, TACE and intra-arterial infusion of chemotherapy directed at the segment of liver containing metastases have all been used when surgery is not indicated, with the aim of reducing disease burden and prolonging survival [27]. TACE has also been used in combination with techniques such as RF ablation, surgical resection and laser-induced thermocoagulation.
A more recently described technique is portal vein embolisation, used to induce hepatic hypertrophy prior to surgical resection of tumour-bearing liver [28–30]. This allows a more extensive hepatic resection, and reduces the rates of post-operative complications including liver dysfunction [29]. The portal vein supplying the tumour-bearing lobe is embolised by percutaneous transhepatic cannulation of the portal vein, with the goal of reduction in volume of normal liver parenchyma in the target distribution. The non-embolised liver segments then undergo hypertrophy which allows extended hepatectomy to be performed. It has been estimated that 25% of final liver volume is the minimum amount required for preserved hepatic function following hepatectomy (40% if the liver is cirrhotic) [30]. For patients who have an estimated residual liver volume below these levels, portal vein embolisation may be performed in order to attempt to increase the residual liver volume and reduce the risk of liver dysfunction post-operatively. This technique has been described both for treatment of colorectal hepatic metastases, and for other hepatobiliary malignancies, including HCC, cholangiocarcinoma and gallbladder carcinoma.
Efficacy
Despite being established as a technique, few data support the use of TAE or TACE for the treatment of colorectal liver metastases. Hunt et al. [31] have published the only RCT comparing embolisation with no treatment; in this study 61 patients were randomised to receive no treatment, TAE or hepatic arterial infusional chemotherapy. No benefit of treatment was seen in the TAE group compared to the untreated group, and a small but non-significant increased survival was noted in the infusion chemotherapy group. Salman et al. [32] evaluated the use of TAE and TACE as second-line therapy in 50 patients with colorectal liver metastases. Median survival for all patients was 11 months, although for patients with metastases solely in the liver, the median survival was 15 months. They concluded that following TAE or TACE median survival is similar to that of other second-line therapies. A similar median survival (9.6 months) was observed by Hunt et al. in patients assigned to receive no treatment. The anti-tumour effect of TACE is supported by another study [33] in which Lang and Brown describe a series of 46 patients treated with TACE; in eight of these the tumour disappeared following treatment and in 11 there was no disease progression for 12 months. Thirty of forty-six (65%) were alive at 12 months and 6/46 (13%) at 5 years. No control group was used and increased survival was not therefore demonstrated. One explanation for the conflicting evidence for the effect of TAE/TACE on survival may be the variation of vascularity seen in hepatic metastases between different patients. Taniai et al. [34] compared the response to TACE between hypovascular and hypervascular tumours, with evidence of response to treatment being seen only in the group with hypervascular tumours.
Chemoembolisation may offer the hope of improved survival when used to compliment other local ablative techniques such as RF ablation or laser-induced thermotherapy (LITT). Vogl et al. [35] have recently published a study of the utility of TACE in reducing the size of hepatic metastases deemed unresectable and too large for LITT alone. Of 162 patients initially treated with TACE, 82 responded (of which 62 were patients with colorectal primary) and became suitable for LITT. Repeated TACE was continued in those not suitable for LITT. Median survival in the combined treatment group was 26.2 months, compared to 12.8 months in the patients who only received TACE, providing evidence for the use of TACE as a neoadjuvant therapy in this setting.
Portal vein embolisation appears to be a safe procedure, and was developed following the observation that portal vein occlusion by tumour or ligation results in hypertrophy of the lobe with intact portal vein supply [30]. It appears to be effective in inducing hypertrophy of the contralateral lobe [29, 30], and results in fewer post-resection complications than are seen in patients who undergo extended hepatectomy [28, 29]. In one study [30], 18 patients who were initially not candidates for surgical resection underwent portal vein embolisation prior to one- or two-stage hepatic resection. The 3-year survival in this group was similar to that of patients who were initially candidates for hepatic resection. Increased tumour growth in the hypertrophied lobe has been described [36], hence patient selection is important to minimise the risk of tumour remaining within the liver remnant.
At present surgical resection remains the only modality of therapy for patients with colorectal liver metastases that prolongs survival; some patients not initially candidates for hepatic resection may be suitable for induced hepatic hypertrophy by portal vein embolisation, followed by hepatic resection, with a resultant increase in survival. Likewise, TAE and TACE may be appropriate in conjunction with another percutaneous therapy. At present no consistent effect of TAE/TACE alone has been shown on survival in patients with colorectal liver metastases.
Hepatic metastases from neuroendocrine tumours
Primary neuroendocrine endocrine tumours of the midgut are slow growing, and generally asymptomatic, as endocrine products secreted by the tumour enter the portal venous system, and are metabolised by the liver. It is usually with the development of liver metastases that the patient becomes symptomatic, as the vasoactive tumour products are now released into the systemic circulation and the patient develops the carcinoid syndrome. For this reason, neuroendocrine tumours tend to present late and at an advanced stage, with liver metastases, and are then incurable. For these patients, treatment is palliative and is performed to relieve symptoms of the carcinoid syndrome and of local bulky disease.
The treatment of these patients is multi-modality, with surgical resection, TAE/TACE and medical therapy with somatostatin analogues all employed. Surgical resection provides good symptomatic relief and prolonged response to treatment, but is only suitable for selected patients, for whom it is estimated that surgery will remove over 90% of the tumour volume [37]. In selected patients, surgical resection may also prolong survival [37, 38]. Patients who are not candidates for surgery will need other modalities of treatment, including embolisation, for palliation.
Efficacy
Many studies have been published that demonstrate good biochemical and symptomatic response to TAE or TACE [38–51], and long-term palliation appears to be effective. The duration of the response to therapy is good, ranging from a median of 11 months [40] to 29 months [42]. In a recent case-series by Roche et al. [50] objective morphological response to treatment with TACE was seen in 12 out of 14 patients, with three successfully palliated for 55, 69 and 100 months from first treatment. Many of the series utilised TAE or TACE in conjunction with medical therapy, such as a somatostatin analogue, interferon or chemotherapy. Roche et al. used TACE as first-line therapy, with similar results to prior studies using multi-modality therapy.
The effect of TAE/TACE on patient survival is less clear; Mitty et al. [39] found survival to be increased following TAE, with mean life-span of 5.4 years from onset of flushing, compared to an expected survival of 3.2 years. In other studies, however, mean survival has varied with reported mean survival times of 16 months [38], 24 months [42, 44], and 40 months [41]. More recently Loewe et al. [51] have published a case-series of 75 embolisations on 23 patients, with median survival of 69 months and 5-year survival of 65%, which compares favourably to 5-year survival in other trials of 53% [38] and 54% [48]. No large, randomised controlled trial exists and so there is no high level evidence of improved survival following TACE in patients with hepatic metastases from neuroendocrine tumours.
It is common for patients with neuroendocrine metastases to develop post-embolisation syndrome, and it appears to be more severe following a first episode of embolisation than following subsequent episodes [51]. Reported mortality varies, with one study [42] having no procedure related deaths out of 23 patients. In other studies mortality related to TAE/TACE ranges from 8.6% [51] to 11% [48]. Two authors report deaths in patients treated with TAE who had tumour volume exceeding 75% of liver volume [48, 51], which suggests that patients with this degree of liver replacement by tumour should not receive TAE/TACE.
Currently, the literature supports the use of TAE or TACE for palliation of patients with hepatic metastases from neuroendocrine tumours when performed to provide relief from symptoms of carcinoid syndrome or of local bulky disease. There has been, as yet, no survival benefit demonstrated, when used both as first-line therapy and in conjunction with medical therapy and/or chemotherapy.
Renal cell carcinoma
Embolisation has been used in the treatment of renal cell carcinoma in the following ways: prior to nephrectomy to facilitate surgery and reduce peri-operative blood loss; as a palliative treatment for large inoperable tumours causing local symptoms or haematuria; for palliation of symptoms due to distant metastases; and in the emergency treatment of life-threatening haemorrhage [52].
Technique
A variety of embolic agents have been described, including soluble gelatine sponge, ethanol, lipiodol, stainless steel coils and Ethibloc, an occlusion gel which causes capillary occlusion. Coils, gelatine sponge and PVA are often used for control of haemorrhage, while a combination of ethanol and coils or PVA may be used for other indications, such as tumour palliation. The differing techniques make comparison between studies difficult, and no treatment protocol has any proven benefit over another.
Efficacy
An early series [53] of 55 patients suggested pre-operative embolisation made nephrectomy easier to perform, but failed to show an influence of embolisation on regression of tumour or metastases. A subsequent series by Bakke et al. [54] showed no difference in survival of patients with stage 4 renal cell carcinoma treated with embolisation/nephrectomy and embolisation alone. Fischedick et al. [55] also found no survival benefit of embolisation prior to nephrectomy, and demonstrated no difference in peri-operative blood loss during nephrectomy between patients who received embolisation and those who did not. The failure of embolisation to reduce peri-operative blood loss was also seen in further series of 35 patients treated with embolisation with ethanol [56]. In contrast, Bakal et al. [57] found embolisation did reduce peri-operative blood loss when the tumour was large and hypervascular. Overall the data do not support routine use of embolisation prior to nephrectomy, and a survey of British urologists on their use of routine embolisation revealed the vast majority did not routinely combine embolisation with nephrectomy [56].
Embolisation is effective at providing symptomatic relief from local disease [58], and there is some evidence that palliative embolisation of inoperable tumours can prolong survival. Onishi et al. [59] published a cohort study comparing two groups of patients with stage 4 renal cell cancer, with one group receiving embolisation with ethanol and the other only symptomatic therapy. The two groups showed no difference in performance status or tumour characteristics, although there was a higher incidence of paraneoplastic syndrome in the group treated with embolisation. Median survival was approximately 7 months following TAE compared with 4 months with symptomatic therapy; the 1-year, 2-year and 3-year survival rates were 29, 15 and 10% in the embolisation group, and 13, 7 and 3% in the non-embolisation group. In the embolisation group, post-embolisation syndrome was common, but no major complications were reported. Five-year survival for stage 4 disease treated surgically is 6% [60]; the patients in the study by Onishi were not suitable for surgery on the basis of poor performance status and would therefore be expected to have a worse prognosis than those in this surgical series. Park et al. [61] described a case-series using a combination of ethanol and lipiodol. Evidence of reduction in tumour volume was seen in 6/14 patients, and median survival was 23 months for stage 3 disease and 7 months for stage 4 disease. In a small case-series, Kauffmann et al. [62] used an occlusion gel to perform capillary occlusion, resulting in apparently spectacular mean survival times of 6 years and 4 months in patients without metastases and 3 years in patients with metastases; no large or controlled study has produced similar figures.
Embolisation of distant metastases for relief of pain has been successful for lesions in the vertebral column [63], limbs [64, 65] and pelvis [65], and in addition to being used for acute haemorrhage from the renal primary, has been used successfully to stop haemorrhage from a renal cell metastasis in the small bowel [66].
Complications
Complications of embolisation for renal cell carcinoma include inadvertent embolisation of non-target arteries, usually the lower extremities, renal failure, renal abscess and post-embolisation syndrome, and occur in approximately 10% of cases. The complication rate is up to four times higher for palliative than pre-operative procedures [67]. Mortality related to the procedure ranges from 0% [56] to 5.8% [68]. Radical nephrectomy has a complication rate of 20% and mortality of 2% [69].
Conclusion
Although numerous studies on TAE/TACE for primary and secondary hepatic tumours and renal cell cancer have been produced, there is a great deal of heterogeneity in the patient selection criteria, treatment protocols and outcome measures used. Furthermore, with the difficulty in producing randomised controlled trials for the interventional techniques used, there remain areas in which the role of embolisation is unclear. Nevertheless, it is possible to define situations in which embolisation is an effective therapy.
There is level 1 evidence that TACE offers patients with non-resectable HCC the chance of prolonged survival. However, because of the potential risk of acute liver failure, patient selection is important, with factors suggesting a poor prognosis being tumour diameter above 10 cm, diffuse or infiltrating tumour pattern, more than nine tumours within the liver and poor baseline liver function [4]. TACE may also prove effective in combination with other modalities of treatment, both as an adjuvant to hepatic resection or transplantation, and in combination with locoregional therapies such as PEI and thermal ablation where the tumour is too large for those techniques alone.
Although TACE has proved disappointing for the treatment of colorectal liver metastases, the technique of portal vein embolisation has been developed to allow extended hepatectomy for patients with hepatic metastases. Although this remains a palliative treatment, it may enable a larger proportion of patients with colorectal liver metastases to undergo hepatic resection with a resultant prolonged survival. TACE may however prove useful in conjunction with thermal ablative techniques in the future.
As with colorectal metastases, TAE/TACE has not been shown to prolong survival for patients with hepatic metastases from neuroendocrine tumours. However, many of these patients suffer from local pain from bulky disease and with symptoms due to vasoactive substance release, and for these patients good symptomatic relief has been shown following treatment with TAE and TACE. Furthermore, long-term palliation with multiple courses of embolisation is possible, with symptom-free survival of many years having been achieved.
Pre-operative embolisation for renal cell carcinoma is not performed routinely, although a few urologists still consider it useful for large vascular tumours. There is little evidence of prolonged survival in this circumstance. When used in the palliation of inoperable renal cell cancer, a number of case-reports and case-series exist in which embolisation has been used to control haemorrhage and provide relief from local and metastatic pain. Despite a single cohort study [57] providing level 2 evidence of prolonged survival following palliative embolisation of renal cell carcinoma, prospective evaluation is still needed in order to clarify the effect of embolotherapy on progression of renal cell carcinoma and survival.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–1994. Lancet. 1997;350:1142–3. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey DE, Kernagis LY, Soulen MC, Geschwind J-FH. Chemoembolization of hepatocellular carcinoma. J Vasc Intervent Radiol. 2002;13:S211–21. doi: 10.1016/s1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind J-FH. Chemoembolization for hepatocellular carcinoma: where does the truth lie? J Vasc Intervent Radiol. 2002;13:991–4. doi: 10.1016/s1051-0443(07)61862-4. [DOI] [PubMed] [Google Scholar]
- 5.Kamada K, Nakanishi T, Kitamoto M, et al. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Intervent Radiol. 2001;12:847–54. doi: 10.1016/s1051-0443(07)61510-3. [DOI] [PubMed] [Google Scholar]
- 6.Llado L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88(1):50–7. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka Y, Yamashita Y, Takahashi M, et al. Unresectable hepatocellular carcinoma: analysis of prognostic factors in transcatheter management. Radiology. 1995;195(3):747–52. doi: 10.1148/radiology.195.3.7754005. [DOI] [PubMed] [Google Scholar]
- 8.Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodised oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88(7):1574–81. [PubMed] [Google Scholar]
- 9.Groupe d’Etude et de Traitment du Carcinome Hepatocellulaire A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995:1256–61. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomised trial in a single institution. Hepatology. 1998;27(6):1578–83. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 11.Pelletier G, Ducreux M, Gay F, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomised trial. Groupe CHC. J Hepatol. 1998;29(1):129–34. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 12.Bayraktar Y, Balkanci F, Kayhan B, et al. A comparison of chemoembolization with conventional chemotherapy and symptomatic treatment in cirrhotic patients with hepatocellular carcinoma. Hepato-gastroenterology. 1996;43(9):681–7. [PubMed] [Google Scholar]
- 13.Lo C-M, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Real MI, Montana X, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 15.Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomised controlled trials. Radiology. 2002;224(1):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bruix J. Systematic review of randomised trials for hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Liu Q, He J, Yang J, Yang G, Wu M. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer. 2000;89(12):2606–12. [PubMed] [Google Scholar]
- 18.Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9(6):557–63. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 19.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197(1):101–8. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Okazaki H, Nakamura S, et al. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179(3):713–7. doi: 10.1148/radiology.179.3.1851313. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Nakamura S, Numata K, et al. Hepatocellular carcinoma: treatment with percutaneous ethanol injection and transcatheter arterial embolization. Radiology. 1992;185(2):457–60. doi: 10.1148/radiology.185.2.1329143. [DOI] [PubMed] [Google Scholar]
- 22.Bartolozzi C, Lencioni R, Caramella D, et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197(3):812–8. doi: 10.1148/radiology.197.3.7480761. [DOI] [PubMed] [Google Scholar]
- 23.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–40. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 24.Pacella CM, Bizzarri G, Celloni P, et al. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219(3):669–78. doi: 10.1148/radiology.219.3.r01ma02669. [DOI] [PubMed] [Google Scholar]
- 25.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94(6):1747–52. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 26.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38(7):1023–33. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 27.Wallace S, Carrasco CH, Charnsangavej C, Richli WR, Wright K, Gianturco C. Hepatic artery infusion and chemoembolisation in the treatment of liver metastases. Cardiovasc Intervent Radiol. 1990;13(3):153–60. doi: 10.1007/BF02575467. [DOI] [PubMed] [Google Scholar]
- 28.Jaeck D, Bachellier P, Nakano H, et al. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg. 2003;185(3):221–9. doi: 10.1016/s0002-9610(02)01373-9. [DOI] [PubMed] [Google Scholar]
- 29.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237(5):686–91. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness—study in 26 patients. Radiology. 2003;227(1):251–60. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 31.Hunt TM, Flowerdew AD, Birch SJ, Williams JD, Mullee MA, Taylor I. Prospective randomised trial of hepatic arterial embolization or infusion chemotherapy with 5-fluorouracil and degradable starch microspheres for colorectal liver metastases. Br J Surg. 1990;77(7):779–82. doi: 10.1002/bjs.1800770720. [DOI] [PubMed] [Google Scholar]
- 32.Salman HS, Cynamon J, Jagust M, et al. Randomized phase II trial of embolization therapy in previously treated patients with colorectal carcinoma metastatic to the liver. Clin Colorectal Cancer. 2002;2(3):173–9. doi: 10.3816/CCC.2002.n.022. [DOI] [PubMed] [Google Scholar]
- 33.Lang EK, Brown CL Jr. Colorectal metastases to the liver: selective chemoembolization. Radiology. 1993;189(2):417–22. doi: 10.1148/radiology.189.2.8210369. [DOI] [PubMed] [Google Scholar]
- 34.Taniai N, Onda M, Tajiri T, et al. Good embolization response for colorectal liver metastases with hypervascularity. Hepato-gastroenterology. 2002;49(48):1531–4. [PubMed] [Google Scholar]
- 35.Vogl TJ, Mack MG, Balzer JO, et al. Liver metastases: neoadjuvant downsizing with transarterial chemoembolization before laser-induced thermotherapy. Radiology. 2003;229(2):457–64. doi: 10.1148/radiol.2292021329. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, De Baere T, Roche A, Mducreux M, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–8. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 37.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumours. Surg Oncol Clin N Am. 2003;12(1):231–42. doi: 10.1016/s1055-3207(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 38.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(4):432–45. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 39.Mitty HA, Warner RR, Newman LH, Train JS, Parnes IH. Control of carcinoid syndrome with hepatic artery embolization. Radiology. 1985;155(3):623–6. doi: 10.1148/radiology.155.3.4001362. [DOI] [PubMed] [Google Scholar]
- 40.Carrasco CH, Charnsangavej C, Ajani J, Samaan NA, Richli W, Wallace S. The carcinoid syndrome: palliation by hepatic artery embolization. AJR Am J Roentgenol. 1986;147(1):149–54. doi: 10.2214/ajr.147.1.149. [DOI] [PubMed] [Google Scholar]
- 41.Hajarizadeh H, Ivancev K, Mueller CR, Fletcher WS, Woltering EA. Effective palliative treatment of metastatic carcinoid tumors with intra-arterial chemotherapy/chemoembolization combined with octreotide acetate. Am J Surg. 1992;163(5):479–83. doi: 10.1016/0002-9610(92)90392-5. [DOI] [PubMed] [Google Scholar]
- 42.Therasse E, Breittmayer F, Roche A, et al. Transcatheter chemoembolization of progressive carcinoid liver metastases. Radiology. 1993;189(2):541–7. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 43.Moertel CG, Johnson CM, McKusick MA, et al. The management of patients with advanced carcinoid tumours and islet cell tumours. Ann Intern Med. 1994;120(4):302–9. doi: 10.7326/0003-4819-120-4-199402150-00008. [DOI] [PubMed] [Google Scholar]
- 44.Perry LJ, Stuart K, Stokes KR, Clouse ME. Hepatic arterial chemoembolization for metastatic neuroendocrine tumours. Surgery. 1994;116(6):1111–6. [PubMed] [Google Scholar]
- 45.Diaco DS, Hajarizadeh H, Mueller CR, Fletcher WS, Pommier RF, Woltering EA. Treatment of metastatic carcinoid tumors using multimodality therapy of octreotide acetate, intra-arterial chemotherapy, and hepatic arterial chemoembolization. Am J Surg. 1995;169(5):523–8. doi: 10.1016/S0002-9610(99)80210-4. [DOI] [PubMed] [Google Scholar]
- 46.Diamandidou E, Ajani JA, Yang DJ, et al. Two-phase study of hepatic artery vascular occlusion with microencapsulated cisplatin in patients with liver metastases from neuroendocrine tumors. AJR Am J Roentgenol. 1998;170(2):339–44. doi: 10.2214/ajr.170.2.9456942. [DOI] [PubMed] [Google Scholar]
- 47.Eriksson BK, Larsson EG, Skogseid BM, Lofberg AM, Lorelius LE, Oberg KE. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumours. Cancer. 1998;83(11):2293–301. [PubMed] [Google Scholar]
- 48.Brown KT, Koh BY, Brody LA, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Intervent Radiol. 1999;10(4):397–403. doi: 10.1016/s1051-0443(99)70055-2. [DOI] [PubMed] [Google Scholar]
- 49.Dejong CH, Parks RW, Currie E, Piris J, Redhead DN, Garden OJ. Treatment of hepatic metastases of neuroendocrine malignancies: a 10-year experience. J R Coll Surg Edinb. 2002;47(2):495–9. [PubMed] [Google Scholar]
- 50.Roche A, Girish BV, de Baere T, et al. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumours. Eur Radiol. 2003;13(1):136–40. doi: 10.1007/s00330-002-1558-0. [DOI] [PubMed] [Google Scholar]
- 51.Loewe C, Schindl M, Cejna M, Niederle B, Lammer J, Thurnher S. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol. 2003;180:1379–84. doi: 10.2214/ajr.180.5.1801379. [DOI] [PubMed] [Google Scholar]
- 52.Roy C, Tuchmann C, Morel M, Saussine C, Jacqmin D, Tongio J. Is there still a place for angiography in the management of renal mass lesions? Eur Radiol. 1999;9(2):329–35. doi: 10.1007/s003300050675. [DOI] [PubMed] [Google Scholar]
- 53.Kaisary AV, Williams G, Riddle PR. The role of preoperative embolization in renal cell carcinoma. J Urol. 1984;131(4):641–6. doi: 10.1016/s0022-5347(17)50556-x. [DOI] [PubMed] [Google Scholar]
- 54.Bakke A, Goethlin J, Hoisaeter PA. Renal malignancies: outcome of patients in stage 4 with or without embolization procedure. Urology. 1985;26(6):541–3. doi: 10.1016/0090-4295(85)90355-3. [DOI] [PubMed] [Google Scholar]
- 55.Fischedick AR, Peters PE, Kleinhans G, Pfeifer E. Preoperative renal tumor embolization. A useful procedure? Acta Radiol. 1987;28(3):303–6. [PubMed] [Google Scholar]
- 56.Lanigan D, Jurriaans E, Hammonds JC, Wells IP, Choa RG. The current status of embolization in renal cell carcinoma—a survey of local and national practice. Clin Radiol. 1992;46(3):176–8. doi: 10.1016/s0009-9260(05)80440-4. [DOI] [PubMed] [Google Scholar]
- 57.Bakal CW, Cynamon J, Lakritz PS, Sprayregen S. Value of preoperative renal artery embolization in reducing blood transfusion requirements during nephrectomy for renal cell carcinoma. J Vasc Intervent Radiol. 1993;4(6):727–31. doi: 10.1016/s1051-0443(93)71958-2. [DOI] [PubMed] [Google Scholar]
- 58.Munro NP, Woodhams S, Nawrocki JD, Fletcher M, Thomas PJ. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92(3):240–4. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- 59.Onishi T, Oishi Y, Suzuki Y, Asano K. Prognostic evaluation of transcatheter arterial embolization for unresectable renal cell carcinoma with distant metastases. BJU Int. 2001;87(4):312–5. doi: 10.1046/j.1464-410x.2001.00070.x. [DOI] [PubMed] [Google Scholar]
- 60.Sene AP, Hunt L, McMahon RF, Carroll RN. Renal cell carcinoma in patients undergoing nephrectomy: analysis of survival and prognostic factors. Br J Urol. 1992;70(2):125–34. doi: 10.1111/j.1464-410x.1992.tb15689.x. [DOI] [PubMed] [Google Scholar]
- 61.Park JH, Kim SH, Han JK, Chung JW, Han MC. Transcatheter arterial embolization of unresectable renal cell carcinoma with a mixture of ethanol and iodised oil. Cardiovasc Intervent Radiol. 1994;17(6):323–7. doi: 10.1007/BF00203951. [DOI] [PubMed] [Google Scholar]
- 62.Kauffmann GW, Richter GM, Rohrbach R, Wenz W. Prolonged survival following palliative renal tumor embolization by capillary occlusion. Cardiovasc Intervent Radiol. 1989;12(1):22–8. doi: 10.1007/BF02577121. [DOI] [PubMed] [Google Scholar]
- 63.Nickolisen R, Fallon B. Locally recurrent hypernephroma treated by radiation therapy and embolization. A report of two cases. Cancer. 1985;56(5):1049–51. doi: 10.1002/1097-0142(19850901)56:5<1049::aid-cncr2820560514>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 64.Stepanek E, Joseph S, Campbell P, Porte M. Embolization of a limb metastasis in renal cell carcinoma as a palliative treatment of bone pain. Clin Radiol. 1999;54(12):855–7. doi: 10.1016/s0009-9260(99)90695-5. [DOI] [PubMed] [Google Scholar]
- 65.Yilmaz S, Sindel T, Luleci E. Repeated palliative embolization of renal cell carcinoma metastases. Clin Radiol. 2002;57(4):855–7. doi: 10.1053/crad.2001.0885. (Letter). [DOI] [PubMed] [Google Scholar]
- 66.Gordon B, Lossof SV, Jelinger E, Barth KH. Embolotherapy for small bowel hemorrhage from metastatic renal cell carcinoma: case report. Cardiovasc Intervent Radiol. 1991;14(5):311–3. doi: 10.1007/BF02578457. [DOI] [PubMed] [Google Scholar]
- 67.Lammer J, Justich E, Schreyer H, Pettek R. Complications of renal tumor embolization. Cardiovasc Intervent Radiol. 1985;8(1):31–5. doi: 10.1007/BF02552637. [DOI] [PubMed] [Google Scholar]
- 68.Kurth KH, Debruyne FM, Hall RR, et al. Embolization and post-infarction nephrectomy in patients with primary metastatic renal adenocarcinoma. Eur Urol. 1987;13(4):251–5. doi: 10.1159/000472789. [DOI] [PubMed] [Google Scholar]
- 69.Swanson DA, Borges PM. Complication of transabdominal radical nephrectomy for renal cell carcinoma. J Urol. 1983;129(4):704–7. doi: 10.1016/s0022-5347(17)52321-6. [DOI] [PubMed] [Google Scholar]