Abstract
Improvements in imaging technology are impacting on every stage of the radiotherapy treatment process. Fundamental to this is the move towards computed tomography (CT) simulation as the basis of all radiotherapy planning. Whilst for many treatments, the definition of three-dimensional (3D) tumour volumes is necessary, for geometrically simple treatments virtual simulation may be more speedily performed by utilising the reconstruction of data in multiple imaging planes. These multi-planar reconstructions may be used to define both the treatment volumes (e.g. for palliative lung treatments) and the organs at risk to be avoided (e.g. for para-aortic strip irradiation). For complex treatments such as conformal radiotherapy (CFRT) and intensity-modulated radiotherapy (IMRT) where 3D volumes are defined, improvements in imaging technologies have specific roles to play in defining the gross tumour volume (GTV) and the planning target volume (PTV). Image registration technologies allow the incorporation of functional imaging, such as positron emission tomography and functional magnetic resonance imaging, into the definition of the GTV to result in a biological target volume. Crucial to the successful irradiation of these volumes is the definition of appropriate PTV margins. Again improvements in imaging are revolutionising this process by reducing the necessary margin (active breathing control, treatment gating) and by incorporating patient motion into the planning process (slow CT scans, CT/fluoroscopy units). CFRT and IMRT are leading to far closer conformance of the treated volume to the defined tumour volume. To ensure that this is reliably achieved on a daily basis, new imaging technologies are being incorporated into the verification process. Portal imaging has been transformed by the introduction of electronic portal imaging devices and a move is underway from two-dimensional (2D) to 3D treatment verification (cone beam CT, optical video systems). A parallel development is underway from off-line analysis of portal images to the incorporation of imaging at the time of treatment using image-guided radiotherapy. By impacting on the whole process of radiotherapy (tumour definition, simulation, treatment verification), these new imaging technologies offer improvements in radiotherapy delivery with the potential for greater cure rates and a minimum level of treatment side effects.
Keywords: Radiotherapy treatment planning, virtual simulation, image registration, treatment verification
Introduction
The process of radiotherapy has been likened to a chain, with the whole process being only as strong as its weakest link. Each of these links (tumour definition, simulation, treatment planning, treatment delivery) can be strengthened and enhanced by improvements in imaging technologies. This paper aims to provide an overview of the role modern imaging plays in radiotherapy planning and delivery, with an eye to the advances on the horizon which may soon enter routine clinical practice.
The conventional simulator
The radiotherapy simulator is a diagnostic X-ray tube, mounted to reproduce the geometric movements of a radiotherapy treatment machine and capable of imaging with both fluoroscopy and plain X-ray film. The imaging produced is intrinsically two-dimensional (2D), and three-dimensional (3D) information can only be obtained by taking orthogonal X-ray films. Whilst still in routine use in radiotherapy, the simulator is rapidly being replaced by the computed tomography (CT) scanner as the standard means of locating the tumour for both radical and palliative radiotherapy treatments. The information derived through CT is inherently 3D and contains both contour and tissue density information which is invaluable during the planning process. Although stand-alone CT scanners may be used, a trend is growing for radiotherapy planning to be performed using dedicated CT simulators. These comprise of a CT scanner, a laser marking system and a 3D workstation to allow the manipulation and visualisation of the CT data for radiotherapy treatment localisation. This process is called virtual (or CT) simulation.
Virtual simulation
Most CT simulators are based upon standard diagnostic CT scanners but as the market has grown, so scanners have been developed which are tailored to the specific needs of radiotherapy. The main feature of these scanners is the increased aperture size (up to 85 cm) allowing the use of patient immobilisation devices not possible with traditional diagnostic scanners (typically 65–70 cm bore). The increased source–detector distance on these larger scanners slightly increases both the noise levels of the scans and the patient dose, but these increases are of the order of a few percent only, and have little diagnostic or clinical impact [1].
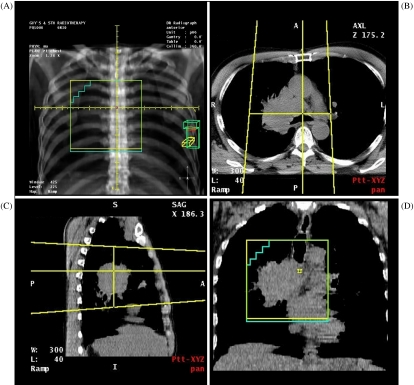
For a thorough description of the computer technology underpinning virtual simulation, see Aird and Conway [2]. In essence, the virtual simulator uses CT data and a 3D computer workstation to replicate the localisation process undertaken in the conventional simulator. This process includes the selection of field sizes, gantry angles and other machine parameters to define treatment beams with the appropriate target coverage. The fluoroscopy image and the X-ray film are replaced by the digitally reconstructed radiograph (DRR). In addition to replicating the functionality of the conventional simulator, the virtual simulator allows visualisation of the field apertures in relation to the 3D CT data. These data may be viewed as conventional axial CT slices or may be reconstructed in other planes, such as coronal or sagittal slices (Fig. 1). This ability to reconstruct the data as multi-planar images (multi-planar reconstructions, MPR) is one of the main advantages of virtual simulation.
Figure 1.
Virtual simulation for non-small cell lung carcinoma (NSCLC). (A) Anterior DRR; (b) axial slice; (C) sagittal MPR; (D) coronal MPR.
Simple field design
The simplest method of virtual simulation is to model the methods used with the conventional simulator. With this approach, the first step is to position the treatment fields on the DRR, and the axial scans and the MPRs are then used to assess the field coverage of the target. A good example of the use of this method is tumour localisation in palliative radiotherapy for non-small cell lung carcinoma (NSCLC). The inadequacies of conventional simulation for localising treatment for this patient group have been demonstrated in a prospective study of 86 patients by McJury et al. [3]. When they compared the fields defined by conventional and virtual simulation, they found a major mismatch in 2D field coverage in 66.2% of patients, and a complete match in only 5.2% of patients. We have performed a study of 10 patients comparing the 3D tumour volume coverage of conventional and virtual simulation for NSCLC. Again it was found that conventional simulation appeared inadequate for a significant number of patients within the group, particularly those with large or medially placed tumours. The localisation method used did not involve the definition of 3D tumour volumes and is demonstrated by Fig. 1. Part (a) shows the field placement on the anterior DRR and parts (b) to (d) show how the tumour volume may be visualised on the axial view and on the coronal and sagittal MPRs. In particular the coronal MPR can be very useful for verifying the position of shielding (in this case with multi-leaf collimator, MLC). In practice, the MPRs are viewed interactively by scrolling through the CT data, allowing an appreciation of the 3D target coverage achieved. However, it must be remembered that the coverage observed is the field coverage, and dosimetric coverage must be inferred from this (as with the conventional simulator).
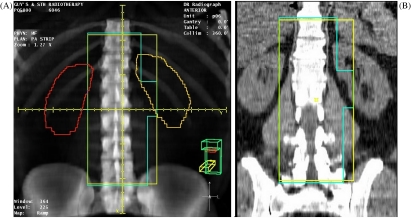
In addition to accurately localising the tumour volume, it is equally important to define any radiosensitive normal tissue structures which may limit the volume to be treated or the dose delivered. These are called the organs at risk (OAR). These organs may be delineated on axial scans to make a 3D volume with appropriate margins, or they may be visualised using the same imaging tools utilised during virtual simulation—namely the DRR, axial scans and MPRs. A good example of OAR which have an impact on the target volume definition are the kidneys in radical prophylactic para-aortic lymph node irradiation for Stage I seminoma of the testis. The treatment aim is to include the para-aortic lymph nodes and the renal hilar nodes on the ipsilateral side, but to exclude both the contralateral and ipsilateral kidneys. Traditionally, these patients are conventionally simulated using IV contrast used to visualise the kidneys. An alternative method of field localisation is to use virtual simulation. Using this method, a full 3D data set is obtained which allows both the more accurate targeting of the treatment area (including the renal hilum) and also visualisation of the kidneys without the use of invasive IV contrast. The localisation of the kidneys may be achieved either by outlining on axial scans to create a composite volume or by interactively visualising them on the coronal MPR (Fig. 2). It may be seen that a comparable view is achieved by both methods, but with a significant time saving using the MPR method.
Figure 2.
Virtual simulation for para-aortic strip irradiation. (A) Anterior DRR with kidneys outlined; (B) coronal MPR with kidneys visualised.
Conformal radiotherapy and intensity-modulated radiotherapy
Recent improvements in radiation oncology technology have enabled greater conformality of the treated volume to the target volume of disease. For many tumour sites it is becoming accepted practice to geometrically shape the treatment beams to deliver a 3D high-dose volume around the tumour whilst avoiding critical OAR nearby. This is termed conformal radiotherapy (CFRT). Intensity-modulated radiation therapy (IMRT) not only shapes the beams geometrically but modulates the fluence of the beams providing improved dose deposition with the possibility of both concave and convex isodose distributions. These techniques have been shown to reduce normal tissue morbidity in randomised clinical trials [4–6] and have the potential for dose escalation to the tumour and improved patient cure [7].
However, in order to implement CFRT and IMRT it is essential that both the tumour volume and any OAR are precisely located and defined. Whilst the localisation of treatment fields without defining target volumes (i.e. using only the DRR, axial scans and MPRs) is acceptable for geometrically simple treatments (parallel opposed fields), it is often not adequate for complex treatment techniques. Here, the accepted approach is to use CT data to localise radiotherapy treatment by outlining the target volume on each axial scan. The union of these 2D volumes over the whole data set results in a 3D target volume. The initial target volume to be drawn is usually the gross tumour volume (GTV), so named as it contains the gross extent of the tumour identifiable with the available clinical and imaging data. To ensure adequate coverage of any sub-clinical (i.e. non discernible) spread, a 3D margin may be added around this GTV to give the clinical target volume (CTV). In turn, a further margin is added around this CTV to account for all the technique-dependent variations such as set-up inaccuracy, internal organ motion and treatment machine parameters, which may result in an inadequate dose coverage of the CTV. The resulting volume is called the planning target volume (PTV). This process is described in detail in the ICRU Report 50 [8]. One advantage of following this procedure is that margins may be added specifically to account for variability due to the treatment site and the imaging modality used for treatment localisation. Against the strong benefits of this system of volumes and margins is the disadvantage of the time-consuming nature of the 3D voluming. Improvement in imaging technology with the development of multi-slice scanners capable of capturing hundreds of axial slices at a time will only add to the time burden of manual volume delineation. The prospect of inherently 3D volume rendering technologies has been raised [9], but currently these are restricted to the definition of OAR and not applicable to tumour volume definition. A novel alternative to defining the tumour volume on each axial slice has been proposed for radical radiotherapy to the prostate. Valicenti et al. [10] investigated the use of ‘thin tissue’ DRRs, where the volume of CT data used is restricted to a 1–1.5 cm section through the proposed target. In this way, the prostate may be clearly visualised against the surrounding normal anatomy and localisation times were reduced to less than 5 min. Whilst this is an interesting approach which mirrors the use of the MPRs for target localisation, in this patient group it did require the administration of both bladder and rectal contrast in order to achieve sufficient tissue definition on the DRRs.
Defining the GTV and OAR
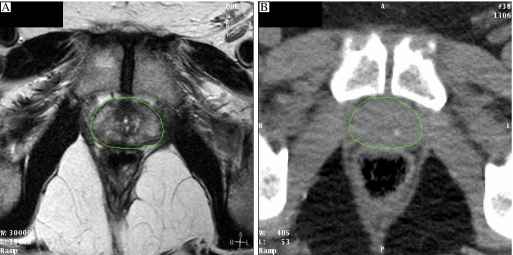
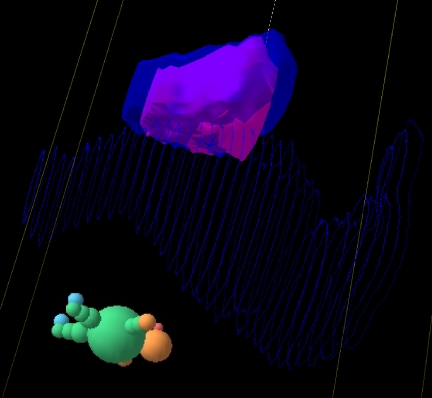
As already described, for reasons of good anatomical visualisation, fast data acquisition and useful tissue density information, the CT scanner has become the foundation of 3D imaging in radiotherapy planning. Whilst for some tumour sites (e.g. lung, bladder, oesophagus) CT is the imaging modality of choice, for other tumour sites it is less well suited. In particular, there are a number of tumour sites where magnetic resonance imaging (MRI) is the optimum modality for delineating the soft tissue extent of tumour. Rasch et al. [11] showed that the impact of incorporating MRI into prostate treatment planning is dramatic with the target volume being on average 30% smaller using MRI compared with CT. The MRI-defined prostate is systematically 7 mm smaller at the posterior aspect (seminal vesicles) and 6 mm at the apex. However, the direct use of MRI for radiotherapy planning has some potentially serious disadvantages: geometric distortion of the image, lack of tissue density information, poor definition of bone, lack of DRR formation and dependency of imaging on scan settings. Despite these problems, attempts have been made to use MRI alone for radiotherapy treatment planning for the prostate. Lee et al. [12] used MRI scans with bulk density information assigned for soft tissue and bone, and showed that there were negligible differences in the dosimetry when compared with CT-based planning. The authors did conclude, however, that more work was needed to define MRI protocols which gave sufficient definition of the prostate whilst minimising geometrical distortion. In order to incorporate MRI data into the planning process, it is therefore necessary to register or fuse the MRI and CT data. Commonly this is achieved through matching the fixed bony anatomy visualised on CT with that seen on MRI. However, the reliability of the image registration depends strongly on the protocols followed for CT and MRI acquisition. Ideally the same immobilisation devices should be used, and any bowel or bladder filling protocols should be applied. Fig. 3 shows the extra detail which may be gained through T2-weighted MRI scans of the prostate compared with the planning CT scan, but it may be noted that rectal filling between the two scans has not been well maintained, leading to potential discrepancies in prostate position within the pelvis. It has been very well established that there is both inter-fraction motion of the prostate between radiotherapy treatments [13] and intra-fraction movement during each treatment [14]. This highlights a significant problem with the use of bony landmarks for MRI/CT registration, as any inter- and intra-fraction motion of the prostate relative to bony anatomy may also occur between the CT and the MRI. The impact of this may be seen in Fig. 4. The larger volume is the CT-defined prostate GTV (solid blue) and within this is seen the MRI-defined prostate GTV (solid pink). The full rectum (wire frame) at the time of the CT scan is seen posterior to the prostate. It is notable that the MRI-defined prostate is in general smaller than the CT-defined prostate but lies more posteriorly at the superior end, probably due to the differences in rectal filling between the two scans (as seen in Fig. 3). An alternative approach to registration using bony anatomy is to perform image registration using implanted intra-prostatic gold grains [15]. This appears to be a very acceptable method of image registration for prostate radiotherapy as these internal fiducial markers are already in common use for treatment verification (see verification section).
Figure 3.
Registered MRI (A) and CT (B) images of the prostate gland. (A) T2 weighted MRI; (B) CT.
Figure 4.
Three-dimensional view of the CT-defined prostate (blue) and MRI-defined prostate (pink). CT-defined rectum is shown as wire frame blue.
In addition to imaging modalities which provide enhanced anatomical information, there is currently an increasing appreciation of the potential benefits of incorporating functional imaging into the localisation process. This imaging may allow progress from physical conformality of the treatment to a level of biological conformality. For example, using positron emission tomography (PET) or nuclear magnetic resonance imaging (NMR), it may be possible to alter the dose levels across the target volume to boost the dose specifically to hypoxic areas of the tumour [16]. Advances are likely to accrue from the use of functional imaging using PET and single photon emission computer tomography (SPECT) scanning at sites such as the lung, head and neck and lymphomas. Again, these images must be fused with CT or segmented MRI data for direct radiotherapy planning [17, 18]. In patients with NSCLC, PET has been shown to define the lymph node target volume more accurately [19], and may lead to a more consistent definition of the GTV [20]. A potential difficulty is the lack of anatomical information contained within PET scans, meaning that image registration with CT can be problematic [21]. For this reason there is a move towards combined PET/CT scanners which remove the need for anatomical registration of the images. The use of combination PET/CT scanners will allow evaluation of direct integration of PET data into the delineation of the GTV at a number of tumour sites to see if it reduces geographical miss and produces better local control and lower tissue morbidity [22]. Already there is some evidence that in patients with head and neck cancer, lung cancer and various pelvic tumours, PET/CT has a significant impact both on the size of tumour volumes and on the degree of variability between clinicians in delineating disease [23]. Additional studies would be helpful to investigate the correlation between true tumour extension on histopathological specimens after surgery and the activity detected on PET scanning and morphological changes seen on CT.
In addition to more accurately localising the tumour volume, PET has also demonstrated its utility in excluding OAR from the treatment volume. Nishioka et al. [24] used fluorine-18-labelled fluorodeoxyglucose (18F-FDG)-PET images co-registered with MRI and CT in a group of 21 patients with nasopharyngeal and oropharyngeal tumours. They found that parotid sparing became possible in 15 of the patients whose upper neck area near the parotid glands was tumour-free on 18F-FDG-PET. Functional MRI (fMRI) has also shown its potential to aid in the definition and exclusion of OAR, particularly during intracranial irradiation. By performing fMRI studies whilst the patient performs set tasks (such as moving their fingers) it may be possible to minimise the possibility of loss of critical neurological functions [25].
Defining the PTV
Once the GTV/CTV have been defined, it then becomes necessary to ensure that a PTV margin is added which will allow confidence in the dosimetric coverage of these target volumes. There are many factors that contribute to this margin, including internal organ motion, daily set-up, machine geometry and the treatment planning system. This section will focus on the quantification and reduction of uncertainties inherent in the localisation process, and the next section will focus on those due to treatment variabilities.
Due to the speed of multi-slice spiral CT scanners, the entire thorax may now be imaged over one to two normal respiratory cycles. Each part of the scan therefore represents a snapshot of the patient’s anatomy and tumour position during a particular phase of their respiratory cycle. As scanners increase in speed so the number of respiratory cycles sampled decreases. This positional uncertainty due to patient respiration may be resolved in either of two ways. The uncertainty may either be reduced (by respiratory control or treatment gating), or carefully measured and incorporated into the planning process. A novel solution to measuring the uncertainty, developed by Sixel et al. [26], is to incorporate a digital fluoroscopy unit into the CT gantry. Their study of 10 patients undergoing radical radiotherapy for NSCLC found that the motion recorded for each patient was unique, and could not be predicted from the position of the tumour within the chest. The group found that a standard PTV margin of 15 mm would often be inadequate in the cranio-caudal direction, and unnecessarily large laterally. It is, however, a necessary requirement of this system that the tumour can be visualised on fluoroscopy. An alternative approach to measuring tumour motion during respiration is to perform multiple CT planning scans. Lagerwaard et al. [27] investigated the use of three ‘slow’ CT scans of the tumour volume and compared them to the data gathered from three fast scans. Each slice of the slow CT scans took 4 s, allowing capture of a whole respiratory cycle. The tumour volumes defined using the slow scans were predictably larger than with the fast scans, but also demonstrated less variability than the volumes delineated on the fast scans.
Whilst for some patient groups (e.g. those with poor lung function), quantifying and incorporating respiratory motion may be acceptable, for other types of treatment the goal must be reduction of the uncertainty. This is particularly true of IMRT treatments where the dose delivered may be built up by many small field segments over a prolonged daily treatment. Motion of the target can potentially disrupt the addition of these individual dose segments leading to an increased dose inhomogeneity across the target. This may be a particular problem where respiratory motion is a factor, such as IMRT treatment to the lung and breast. A potential solution to this problem is to gate the treatment in time with the respiratory cycle of the patient. This may be done by either monitoring the respiratory cycle, or by restricting respiration mechanically. The latter option includes a range of active breathing control (ABC) devices, which have shown a surprising degree of patient compliance and have been used particularly effectively for breast radiotherapy. An important additional advantage of this approach for breast radiotherapy is the reduction of both lung and heart dose by elevation of the breast away from underlying structures during deep inspiration [28]. In addition, in lung radiotherapy, the diagnostic quality of the imaging is improved by gaining CT information whilst the patient is in breath hold. Due to the increasing capability of modern linear accelerators to achieve beam stability over small numbers of monitor units, gating the treatment in time with the patient’s natural respiratory cycle is now a possibility. A device similar to that used for ABC may be used, but increasingly interest is being shown in the possibility of using computer imaging to monitor the respiration of the patient with no physical intervention. Fig. 5 shows a view captured from a video-based system capable of capturing real-time views of the patient during respiration. Limits may be defined on the acceptable phases of the respiratory cycle for the treatment to be delivered and the software may then gate the treatment delivery automatically. Whilst this may not be applicable in a dynamic IMRT setting, it may be useful for step and shoot IMRT or treatment utilising conventional static fields.
Figure 5.
Real-time capture of patient contour during simulation. Courtesy of Vision RT Limited.
Treatment verification
Effective radiotherapy depends upon consistently and reliably irradiating the CTV to the curative prescribed dose and reducing the irradiation of the OAR to an acceptable level. These two goals may be in conflict and the definition of a large CTV-PTV margin to ensure good CTV coverage will often lead to an unacceptable dose to the OAR. It is therefore of primary importance to ensure both the accuracy of this margin and that it is reduced to the minimum possible. A significant component of this margin is the positional uncertainty due to daily set-up variations, and this uncertainty can be both quantified and minimised through effective treatment verification.
A traditional approach is to use the exit of the radiotherapy treatment beam to produce imaging of the field isocentre. Due to the geometrical compatibility of the treatment machine and the simulator, these films may be compared directly with the simulator films or DRRs. More recently, X-ray film has been replaced by electronic portal imaging devices (EPID) which allow a real-time visualisation of portal views. Both of these methods utilise comparison of the position of bony anatomy within the treatment field, but due to the predominance of Compton scatter rather than the photoelectric effect at treatment energies, bone may be poorly visualised.
An alternative approach is to use implanted gold grain fiducial markers, for instance in the prostate gland, which may be more easily visualised on portal imaging [29]. It is a necessary assumption of this technique that the position of the gold grains inside the prostate remains constant. This has been verified by comparing the relative position of three implanted gold grains in 11 patients over the course of their radical prostate radiotherapy [30]. It was found that the average seed movement was less than 1.5 mm and in those patients where it was significantly greater (three patients), this was due to shrinkage of the prostate over the course of treatment. By incorporating a diagnostic X-ray system into the linear accelerator gantry mounting, it is possible that the position of implanted markers may be tracked in real-time and used to gate the radiotherapy treatment. Shirato et al. [31] showed that using such a system for radical lung treatments, it was possible to reduce the range of tumour motion during radiotherapy from 9.6–38 mm (during normal respiration) to 2.5–5.3 mm (when gated). Cone beam CT is a further development designed to take advantage of the addition of kilovoltage imaging into the treatment room. Through this technique, volumetric images of the treatment site may be gathered from one rotation of the gantry mounting. Although still early in the development of these systems, they offer the possibility of full 3D treatment verification whilst the patient remains on the treatment couch. These new imaging technologies are now grouped under the generic term of image-guided radiotherapy (IGRT).
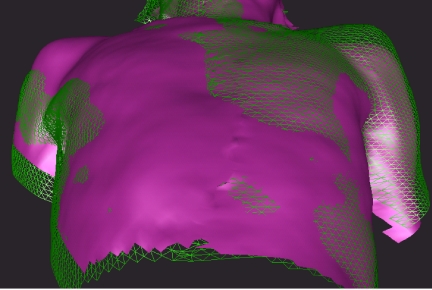
While much of the drive towards IGRT has been through the use of X-ray-based imaging (both megavoltage and kilovoltage), there is a separate approach utilising optical imaging of external markers, or in some systems the whole patient contour. Whilst these systems have the common drawback that the relationship between external markers and internal tumour position must be inferred, they benefit in the lack of additional patient dose and the speed and ease of image acquisition. These systems may additionally be useful during the initial process of patient positioning on the couch. Fig. 6 shows a view derived from a video-based system, with the surface data from the simulator shown in pink and the treatment room shown in green. As the patient positions converge, so this may be easily visualised on the real-time display.
Figure 6.
Real-time alignment of 3D patient contour. Pink is patient contour at simulation; green wire frame is patient contour at treatment. Courtesy of Vision RT Limited.
Conclusion
The wealth of ’state of the art’ imaging that is now available to define the GTV has to be harnessed accurately in order to ensure that it is used to improve patient cure using highly sophisticated CFRT and IMRT. New advances in technology such as multileaf collimation, electronic portal imaging, combined PET/CT scanners and cone beam CT megavoltage treatment units are all adding to the promise of further improvements in the future.
References
- 1.Garcia-Ramirez JL, Mutic S, Dempsey JF, Low DA, Purdy JA. Performance evaluation of an 85 cm bore computed tomography scanner designed for radiation oncology and compared with current diagnostic CT scanners. Int J Radiat Oncol Biol Phys. 2002;52(4):1123–31. doi: 10.1016/s0360-3016(01)02779-1. [DOI] [PubMed] [Google Scholar]
- 2.Aird EG, Conway J. CT simulation for radiotherapy treatment planning. Br J Radiol. 2002;75:937–49. doi: 10.1259/bjr.75.900.750937. [DOI] [PubMed] [Google Scholar]
- 3.McJury M, Fisher PM, Pledge S, et al. The impact of virtual simulation in palliative radiotherapy for non-small cell lung cancer. Radiother Oncol. 2001;59:311–18. doi: 10.1016/s0167-8140(01)00308-5. [DOI] [PubMed] [Google Scholar]
- 4.Dearnaley DP, Khoo VS, Norman AR, et al. Comparison of radiation side effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;353(9149):267–72. doi: 10.1016/S0140-6736(98)05180-0. [DOI] [PubMed] [Google Scholar]
- 5.Dearnaley DP, Hall E, Jackson C, et al. Phase III trial of dose escalation using conformal radiotherapy in prostate cancer: side effects and PSA control. Br J Cancer. 2001;85(Suppl 1):15. doi: 10.1038/sj.bjc.6602301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koper PC, Stroom JC, van Putten WL, et al. Acute morbidity reduction using 3DCRT for prostate carcinoma: a randomized study. Int J Radiat Oncol Biol Phys. 1999;43(4):727–34. doi: 10.1016/s0360-3016(98)00406-4. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166(3):876–81. [PubMed] [Google Scholar]
- 8.Landberg T, Chavaudra J, Dobbs HJ, et al. ICRU Report 50. Prescribing, Recording and Reporting Photon Beam Therapy. International Commission on Radiotherapy Units and Measurements. 1993 [Google Scholar]
- 9.Suit H. The Gray lecture 2001: coming technical advances in radiation oncology. Int J Radiat Oncol Biol Phys. 2002;53:798–809. doi: 10.1016/s0360-3016(02)02851-1. [DOI] [PubMed] [Google Scholar]
- 10.Valicenti RK, Waterman FM, Croce RJ, et al. Efficient CT simulation of the four-field technique for conformal radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:953–7. doi: 10.1016/s0360-3016(96)00568-8. [DOI] [PubMed] [Google Scholar]
- 11.Rasch C, Barillot I, Remeijer P, et al. Definition of the prostate in CT and MRI: a multiobserver study. Int J Radiat Oncol Biol Phys. 1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee YK, Bollet M, Charles-Edwards G, et al. Radiotherapy treatment planning of prostate cancer using magnetic resonance imaging alone. Radiother Oncol. 2003;66:203–16. doi: 10.1016/s0167-8140(02)00440-1. [DOI] [PubMed] [Google Scholar]
- 13.Dawson LA, Mah K, Franssen E, Morton G. Target position variability throughout prostate radiotherapy. Int J Radiat Oncol Biol Phys. 1998;42(5):1155–61. doi: 10.1016/s0360-3016(98)00265-x. [DOI] [PubMed] [Google Scholar]
- 14.Padhani AR, Khoo VS, Suckling J, et al. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999;44(3):525–33. doi: 10.1016/s0360-3016(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 15.Parker CC, Damyanovich A, Haycocks T, Haider M, Bayley A, Catton CN. Magnetic resonance imaging in the radiation treatment planning of localised prostate cancer using intra-prostatic fiducial markers for computed tomography co-registration. Radiother Oncol. 2003;66:217–25. doi: 10.1016/s0167-8140(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 16.Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47(3):551–60. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 17.Van Herk M., The role of multimodality imaging in radiotherapy. Radiother Oncol 2000; 56: Suppl 1, Abstract 53 (presented at ESTRO 2000)
- 18.Rosenman JG, Miller EP, Tracton G, Cullip TJ. Image registration: an essential part of radiation therapy treatment planning. Int J Radiat Oncol Biol Phys. 1998;40(1):197–205. doi: 10.1016/s0360-3016(97)00546-4. [DOI] [PubMed] [Google Scholar]
- 19.Erdi YE, Rosenweig K, Erdi AK, et al. Radiotherapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET) Radiother Oncol. 2002;62:51–60. doi: 10.1016/s0167-8140(01)00470-4. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell CB, Mah K, Ung YC, et al. Observer variation in contouring gross tumour volume in patients with poorly defined non-small cell lung tumours on CT: the impact of 18FDG-hybrid PET fusion. Int J Radiat Oncol Biol Phys. 2001;51:923–31. doi: 10.1016/s0360-3016(01)01722-9. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths M, PET/CT The future’s bright, the future’s fusion. Synergy 2003; November
- 22.Mah K, Caldwell CB, Ung YC, et al. The impact of (18)FDG-PET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small cell lung carcinoma: a prospective study. Int J Radiat Oncol Biol Phys. 2002;52:339–50. doi: 10.1016/s0360-3016(01)01824-7. [DOI] [PubMed] [Google Scholar]
- 23.Ciernik F, Dizendorf E, Baumert B, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57(3):853–63. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka T, Shiga T, Shirato H, et al. Image fusion between FDG-PET and MRI/CT for radiotherapy planning of oropharyngeal and nasopharyngeal carcinomas. Int J Radiat Oncol Biol Phys. 2002;53(4):1051–7. doi: 10.1016/s0360-3016(02)02854-7. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Alvarez R, Liney GP, Beavis AW. Use of functional magnetic resonance imaging in the treatment planning of intensity modulated radiotherapy. J Radiother Practice. 2003;3:55–62. [Google Scholar]
- 26.Sixel KE, Ruschin M, Tirona R, et al. Digital fluoroscopy to quantify lung tumor motion: potential for patient-specific planning target volumes. Int J Radiat Oncol Biol Phys. 2003;57(3):717–23. doi: 10.1016/s0360-3016(03)00713-2. [DOI] [PubMed] [Google Scholar]
- 27.Lagerwaard FJ, Van Sornsen de Koste JR, Nijssen-Visser MRJ, et al. Multiple “slow” CT scans for incorporating lung tumor mobility in radiotherapy planning. Int J Radiat Oncol Biol Phys. 2001;51(4):932–7. doi: 10.1016/s0360-3016(01)01716-3. [DOI] [PubMed] [Google Scholar]
- 28.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55(2):392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 29.Herman MG, Pisansky TM, Kruse JJ, et al. Technical aspects of daily online positioning of the prostate for three-dimensional conformal radiotherapy using an electronic portal imaging device. Int J Radiat Oncol Biol Phys. 2003;57(4):1131–40. doi: 10.1016/s0360-3016(03)00766-1. [DOI] [PubMed] [Google Scholar]
- 30.Pouliot J, Aubin M, Langen KM, et al. (Non) Migration of radiopaque markers used for on-line localisation of the prostate with an electronic portal imaging device. Int J Radiat Oncol Biol Phys. 2003;56(3):862–6. doi: 10.1016/s0360-3016(03)00267-0. [DOI] [PubMed] [Google Scholar]
- 31.Shirato H, Shimizu S, Kunieda T, et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(4):1187–95. doi: 10.1016/s0360-3016(00)00748-3. [DOI] [PubMed] [Google Scholar]