Abstract
Radiotherapy is a localised treatment. The definition of tumour and target volumes for radiotherapy is vital to its successful execution. This requires the best possible characterisation of the location and extent of tumour. Diagnostic imaging, including help and advice from diagnostic specialists, is therefore essential for radiotherapy planning. There are three main volumes in radiotherapy planning. The first is the position and extent of gross tumour, i.e. what can be seen, palpated or imaged; this is known as the gross tumour volume (GTV). Developments in imaging have contributed to the definition of the GTV. The second volume contains the GTV, plus a margin for sub-clinical disease spread which therefore cannot be fully imaged; this is known as the clinical target volume (CTV). It is the most difficult because it cannot be accurately defined for an individual patient, but future developments in imaging, especially towards the molecular level, should allow more specific delineation of the CTV. The CTV is important because this volume must be adequately treated to achieve cure. The third volume, the planning target volume (PTV), allows for uncertainties in planning or treatment delivery. It is a geometric concept designed to ensure that the radiotherapy dose is actually delivered to the CTV. Radiotherapy planning must always consider critical normal tissue structures, known as organs at risk (ORs). In some specific circumstances, it is necessary to add a margin analogous to the PTV margin around an OR to ensure that the organ cannot receive a higher-than-safe dose; this gives a planning organ at risk volume. This applies to an organ such as the spinal cord, where damage to a small amount of normal tissue would produce a severe clinical manifestation. The concepts of GTV, CTV and PTV have been enormously helpful in developing modern radiotherapy. Attention to detail in radiotherapy planning is vital, and does affect outcomes: ‘the devil is in the detail’. Radiotherapy planning is also dependent on high quality imaging, and the better the imaging the better will be the outcomes from radiotherapy.
Keywords: Tumour volume, target volume, conformal radiotherapy, ICRU, organs at risk
Introduction
The purpose of considering how to define tumour and target volumes for radiotherapy is to optimise this treatment modality. Radiotherapy is a speciality which requires attention to detail in order to achieve the best results. It is also important to place radiotherapy in the context of cancer treatments to fully appreciate its value.
The role of radiotherapy in the treatment of cancer
Radiotherapy plays a key role in the management of patients with cancer. After surgery, radiotherapy is the most effective curative treatment for cancer. In 2002, the Cancer Services Collaborative suggested that radiotherapy alone is responsible for 78% of non-surgical cancer cures. Between 30 and 40% of the population will develop cancer, and at least half require radiotherapy at some time in their illness. Of patients having radiotherapy, about 60% are treated with curative intent, often in combination with surgery and chemotherapy. In addition, radiotherapy has an important role in the palliation of symptoms from cancer.
Technological developments in radiotherapy are continuing apace, and are likely to confer further clinical benefit. For example, the Swedish Council on Technology Assessment in Health Care estimated in 1996 that the overall 5-year survival in patients receiving radiotherapy should be increased by approximately 10% over the 10 years following their report as the result of technical innovation, which includes imaging [1].
Fundamental principles of radiotherapy
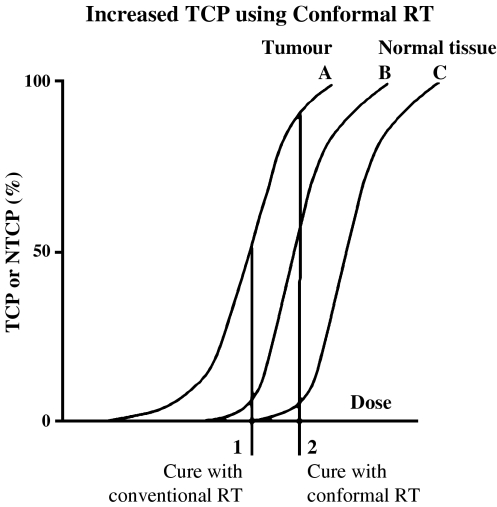
There are three fundamental axioms of radiotherapy which are relevant background to this topic. Firstly, an increase in radiotherapy dose to the tumour normally improves the probability of local control. Secondly, improving local control in the context of a localised tumour achieves an improvement in the overall cure rate, because metastatic spread from local recurrence is avoided. Finally, sparing normal tissues improves the side effect profile of radiotherapy, and hence the therapeutic ratio [2, 3]. Implementation of techniques to spare normal tissue, such as conformal radiotherapy (vide infra), therefore allows a choice between (i) dose increase with a consistent level of side effects, or (ii) the same dose with reduced side effects (see Fig. 1).
Figure 1.
Diagrammatic plot of tumour control probability (TCP) or normal tissue complication probability (NTCP) vs. radiotherapy dose. Sparing normal tissues shifts the NTCP curve to the right (B to C), allowing a lower incidence of normal tissue damage for the same dose (dose 1) or the same level of NTCP for a higher dose (dose 2). This is the basis for an improvement in the therapeutic ratio. This benefit can be the result of any measure which reduces the normal tissue dose, for example including better target imaging, conformal radiotherapy, and improved patient immobilisation.
In fact, rather modest changes in dose both to the tumour and normal tissue can deliver a clinical advantage. For tumours, the effect of a dose increase can be assessed from the slope of the plot of tumour control probability (TCP) vs. radiotherapy dose. In the central part of the curve, where TCP is 50%, the curve is relatively straight. A parameter which describes the percentage increase in TCP for a 1% increase in dose, known as the Gamma-50 (γ50) factor, can be a useful way of estimating the benefit of dose escalation. There is a large amount of literature on both laboratory and clinical data suggesting that a 1% increase in dose typically delivers a 1–2% increase in TCP. Thus, a 10% dose increase, which is realistically achievable in many settings, could achieve an improvement in tumour control of 10–20%. This was achieved in a radiotherapy trial for head and neck cancer, where a 15% dose increase raised the local control from 40 to 59% at 5 years; this indicates a value for γ50 of 1.33 [4].
For normal tissue, a difference in dose to the breast of approximately 10%, within the context of a radiotherapy dose trial, achieved a demonstrable change in the incidence of shape change [5]. The fact that a 10% dose difference led to a 16% difference (24 vs. 40%) in the number of women exhibiting a change in breast appearance at 5 years indicates that modest dose changes produce a clinical effect. This will usually apply whether the dose reduction is to the whole organ, such as the breast, or part of an organ, such as can be achieved with conformal radiotherapy. Thus, even a modest dose increase to the tumour and a dose decrease to normal tissue achieved using advanced radiotherapy planning are worth pursuing.
However, in radiotherapy, tumour and normal tissue are inextricably linked. When delivering a tumoricidal dose to the tumour, some normal tissue is inevitably irradiated. If normal tissue is spared, in dose or volume, the incidence and severity of complications are reduced. This shifts the dose-response curve for complication to the right, allowing a choice between the same dose and tumour control with reduced side effects, and a higher dose with increased tumour control and the same level of normal tissue (see Fig. 1).
This preamble is intended to show that quite small changes in radiotherapy can achieve a significant alteration in clinical outcome. Therefore, attention to the detail of radiotherapy planning is central to the current and improving therapeutic ratio.
Radiotherapy as a localised cancer treatment
Radiotherapy is, by its nature, a localised treatment for localised tumours and in this respect is analogous to surgery. Total body irradiation is an apparent exception, but it is designed to treat all bone marrow and circulating blood, and is a localised treatment to this whole-body target. Thus, disease staging is crucial in the decision on whether to employ radiotherapy as part of the treatment strategy. Better imaging produces better staging, and leads to stage migration which actually gives the illusion of improving outcomes for all tumour stages. Staging is also relevant in defining the extent of radiotherapy, for example whether to include loco-regional lymph nodes.
Following staging, radiotherapy planning requires very specific definition of the location and extent of the primary tumour. Information is also required about the extent of spread around the tumour itself, and the location of regional lymph node spread. Knowledge of anatomy and an understanding of the pathology of tumour spread are essential.
It is important for radiation oncologists to recognise that diagnostic radiologists and radiographers can play an important role in helping to define optimum scanning protocols for use in radiotherapy planning.
It is clear that diagnostic imaging guides the definition of the tumour extent in almost all sites. However, for a successful outcome after radiotherapy, every single tumour cell must be eradicated, including those which have invaded beyond gross, palpable or imageable disease. In radiotherapy planning, therefore, a margin must be added to account for this microscopic spread. This margin extends outside the gross tumour, sometimes for a surprisingly large distance. Here is the fundamental difference between the extent of tumour assessed by current diagnostic cancer imaging and the final definition of a target volume for radiotherapy planning.
Conformal radiotherapy—a definition
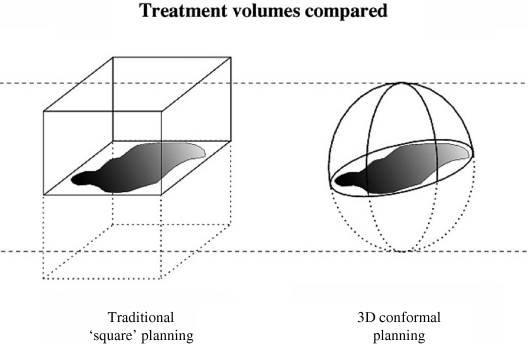
The concept of conformal therapy is to conform the high dose volume as accurately as possible to the target shape, which is an intuitively obvious concept. Broadly, conformal therapy employs geometric field shaping to follow the shape of the target. This is the obvious part. The older ‘square’ style of radiotherapy planning, which pre-dates 3D computer systems, required the use of orthogonal radiographs to delineate the furthest extent of the tumour or target in relation to the bony anatomy of the patient. This would produce six points, representing the furthest extent in each dimension. However, generally no attempt could be made to shape the target, so that these six points define a cuboid structure. Since most tumours grow with a pattern more like a sphere or spheroid, the inclusion of the extra normal tissue in the corners of the cube simply leads to irradiation of (much) more normal tissue to the full target dose. The conformation of treatment volume to the shape of the target quite simply eliminates, or at least reduces, the radiation exposure of a large volume of normal tissue (see Fig. 2).
Figure 2.
The shape of the treatment volume from two techniques of radiotherapy planning. On the left is a cuboidal shape based on old-fashioned ‘square’ planning from orthogonal radiographs; on the right is a spherical shape produced from conformal planning. The two volumes are designed to treat the same tumour target, but the sphere is half the volume of the cube.
This seems to imply that the introduction of conformal radiotherapy has led to a reduction in radiotherapy volumes, and generally this has been the case. However, early experience with computed tomography (CT)-based planning showed that in some circumstances, such as bladder cancer, volumes actually increased, compared to the older-style ‘square’ orthogonal localisation, because the tumour was visualised and localised better.
Further radiotherapy developments, such as intensity-modulated radiotherapy (IMRT), are beyond the scope of this article. However, IMRT is a form of conformal radiotherapy, so the same principles apply.
Definitions for radiotherapy planning
During the 1990s, developments in radiotherapy technology, especially computer technology which introduced the potential to plan radiotherapy dose in three dimensions, led to the recognition that concise definitions of both the primary tumour and possible areas of local spread were required [6, 7]. As well as the obvious application to planning radiotherapy for individual patients, definitions of target volumes are essential for radiotherapy protocols in multi-centre trials where uniformity in planning is required, and to allow reporting of results from different centres.
Although strict definitions might have been usable with the older ‘square’ style of planning, in the era before modern imaging or radiotherapy planning systems the uncertainties in target localisation and dose were so large that such definitions would have offered little advantage.
Now that we can use diagnostic quality imaging directly in radiotherapy planning, imported electronically, it is possible to treat the tumour reliably (i.e. ‘hit the target’) whilst avoiding normal tissue. To maximise the advantages of modern systems we must be very clear in the specification of the radiotherapy target. Sophisticated planning requires robust thinking during the planning process, with adherence to planning protocols, whether local or multi-centre.
Successful radiotherapy also requires the patient to be positioned reliably and reproducibly with respect to the treatment machine, normally a linear accelerator. Considerable effort and subtlety are needed to achieve this goal. During radiotherapy planning, it is essential to know the position of the patient in 3D space with considerable accuracy. This adds a level of difficulty and is different from standard diagnostic imaging. Ultimately, the radiotherapy plan must include information to correctly locate the patient on the linac. The most straightforward method is to locate the patient so that the isocentre of the linac, i.e. its centre of rotation, lies within the target.
Specific target volumes for radiotherapy planning
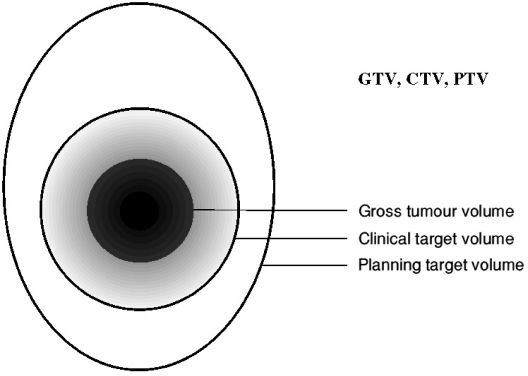
There are three main volumes to be considered in radiotherapy planning, though only the first two of these volumes are of real interest to diagnostic colleagues (see Fig. 3). The first of these two volumes is the position and extent of the primary tumour; this is known as the gross tumour volume (GTV). The second volume surrounds the GTV and describes the extent of microscopic, un-imageable tumour spread; this is known as the clinical target volume (CTV). Once these two volumes are established, the third volume, the planning target volume (PTV), which allows for uncertainties in planning or delivery, must be added, and the normal tissue structures in the vicinity of the target must be considered.
Figure 3.
Diagram to illustrate the main radiotherapy planning volumes, taken from ICRU Report 50.
The original concepts of the GTV and CTV were detailed in Report 50 from The International Commission of Radiation Units and Protection (ICRU) in 1993 [6]. The report also described the principle of the margin needed for uncertainty in the process of planning and delivery, i.e. the PTV. The concept of the PTV was refined in ICRU Report 62 in 1999 [7], and this added further information about organs at risk (ORs) and the need, in certain specific circumstances, to add a margin for uncertainty around an OR to produce a planning organ at risk volume (PRV). Interestingly, Report 62 was specifically triggered by the increasing availability of conformal radiotherapy, where margins are more critical, and the need to describe normal tissue doses in more detail. These two reports set out an underlying philosophy for prescribing, recording and reporting radiotherapy, which has been essential for the successful development of modern radiotherapy.
The PTV was reviewed in 2003 by an expert group under the auspices of the British Institute of Radiology (BIR) [8]. Their report details how to calculate the PTV margins required, and gives worked examples for a number of sites. This report has been most valuable. These volumes are best discussed in a little more detail.
GTV
This is the easiest volume to define, though not necessarily to localise. The GTV is essentially the gross demonstrable location and extent of tumour. It is what can be seen, palpated or imaged (see Fig. 4). As well as a primary site, gross tumour involving lymph nodes or spread into adjacent soft tissue should be included in the GTV. Typically, it is considered that the GTV corresponds to the part of the tumour where the tumour cell density is highest. This may have implications for choice of radiotherapy dose, since tumour control requires a higher dose if the initial tumour cell number is larger.
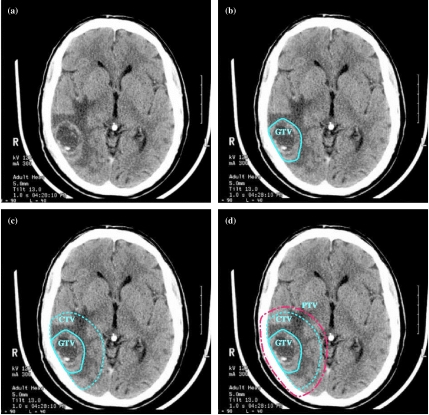
Figure 4.
Planning volumes for a patient with WHO Grade 4 glioma (glioblastoma). (a) Planning CT showing contrast-enhancing tumour. (b) The GTV is the visible tumour. (c) A margin for microscopic spread has been added to make the CTV; the margin is the same in all directions except that it is restricted by the skull. (d) The PTV has been added outside the CTV to account for uncertainties in planning and execution of treatment; this extends beyond the inner table of the skull.
In the post-operative setting where the tumour has been excised, the GTV is no longer evident. Although it is not necessary to attempt to outline a GTV if the tumour has been resected, the position of the CTV must be derived from the site of the original GTV. Therefore, some method of reconstruction of the original tumour is necessary. This applies to rare tumours such as soft tissue sarcomas (Fig. 5), but also to common cancers such as breast cancer, where localisation of the tumour bed may be important. Modern developments in imaging and image matching are sure to be of help in the future.
Figure 5.
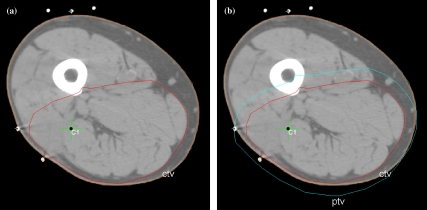
CT planning scan for a patient with a soft tissue sarcoma of the (anatomical) posterior compartment of the thigh. The tumour has been resected so no GTV exists. (a) The CTV is shown, restricted anteriorly by the femur and intermuscular septa. (b) The PTV has been added outside the CTV and in places extends beyond the outside of the patient.
Although conceptually the GTV is usually the easiest to define, in practice the edges of the GTV are not necessarily always clear. Better imaging to delineate gross tumour would be helpful. It is also clear that different imaging modalities may contribute different aspects of GTV localisation. For example, CT and magnetic resonance imaging (MRI) may be complimentary, and positron emission tomography (PET) may for example help to differentiate normal nodes from those involved by tumour.
CTV
The CTV contains the demonstrable GTV plus a margin for sub-clinical disease spread. On occasion, there may be a second CTV, for example in a regional lymph node, where there is no obvious GTV present. The CTV is important because this volume must be adequately treated if cure is to be achieved. It is assumed that the tumour cell density in the CTV is lower than in the GTV and consequently the radiotherapy dose may be lower.
Typically, the CTV margin cannot be fully imaged. It is perhaps the most difficult of all the margins because it requires clinical assessment of risk and extent of spread, normally based on historical series rather than the extent of tumour quantified in an individual patient. For example, for high-grade gliomas it is standard practice to apply a uniform margin around the gross tumour to account for microscopic infiltration, regardless of individual considerations. This margin is based on biopsy and post-mortem series, and is large enough to encompass the maximum extent of invasion seen in (almost) any patient (see Fig. 4).
The extent of the CTV margin depends upon imaging techniques: as these have got more effective at localising gross tumour, so the edge of the gross tumour has typically expanded and the CTV margin contracted, though the final size of the CTV may have remained the same. The CTV is also the biggest area in which developments in diagnostic imaging, especially molecular imaging techniques, will enhance radiotherapy planning.
Typically, the spread of tumours is restrained by some anatomical barriers, and it is important that this information is used to modify the CTV during the process of radiotherapy planning. For example, gliomas normally do not penetrate the skull, so the CTV can be constrained within it (see Fig. 4). Soft tissue sarcomas of the limb are restricted in their axial spread by inter-muscular septa, so the CTV does not have to extend beyond them (see Fig. 5). Additionally, the CTV does not have to extend beyond the surface of the patient.
In some tumour sites it is a definite advantage to be able to use more than one imaging modality to plan radiotherapy. CT is normally used as the basis for radiotherapy planning for a number of reasons. Firstly, it contains density information which can be used to calculate treatment beam attenuation, which improves the accuracy of dosimetry calculations. Secondly, it is reliable in representing shape and position, including the accurate position of fiducial markers. These are used to locate the patient with respect to the treatment machine, are applied to the outside of the patient, and in an MRI could be subject to distortion because they lie in the least homogeneous part of the magnetic field. Finally, the patient can usually be positioned in the CT scanner in the treatment position, which is not possible with MRI. For example, treatment of the chest typically requires the patient to be positioned with the thorax elevated on a wedge-shaped board and the arms above the head, which can be accommodated in a CT. A similar argument applies to a number of other sites, including the breast.
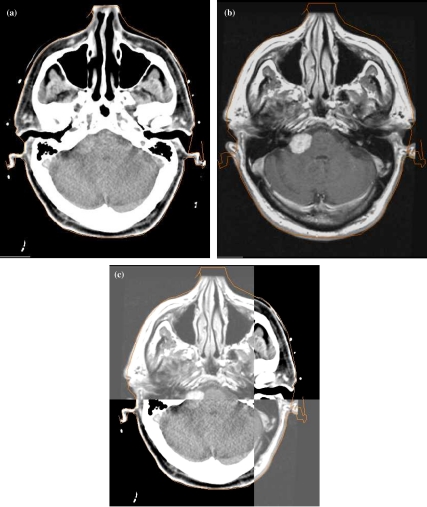
Clearly, additional information may be available from other imaging modalities, especially MRI. The most robust and reliable way to incorporate MRI data into the radiotherapy planning process is to electronically co-register the data and introduce this directly into the planning system. An example is shown in Fig. 6. It is likely that additional imaging will need to be introduced for direct use in planning, including newer MRI sequences, MR spectroscopy, PET imaging, and later molecular imaging. Some development in co-registration techniques is likely to be needed for these.
Figure 6.
Example of the value of image co-registration for radiotherapy planning, which allows planning based directly on the MRI data. (a) Radiotherapy planning CT scan showing right acoustic schwannoma. (b) Diagnostic MRI, which shows the schwannoma clearly. (c) Electronic co-registration of the diagnostic MRI with the planning CT within the radiotherapy planning system. The intersection point can be moved as necessary.
Occasionally, where different imaging modalities are used, there may be uncertainties in the accuracy of co-registration of the modalities. If one modality is displaced relative to the other, provided the size and direction can be measured or estimated, this can be corrected at this stage, or allowed for in the CTV. However, if the size and direction of a discrepancy are unclear or unknown, they should be addressed as part of the PTV (see below). This can be summarised thus: ‘if there is certainty about the uncertainty (of co-registration), it can be dealt with in the CTV; if there is uncertainty about the certainty (of the co-registration), it should be addressed as part of the PTV’.
PTV
The PTV is really a geometric concept designed to ensure that the radiotherapy prescription dose is actually delivery to the CTV. It is a volume related to the isocentre of the linear accelerator rather than to the anatomy of the patient. For this reason, the PTV may extend beyond anatomical barriers such as bony margins, and may even extend outside the patient (Figs 4 and 5).
In ICRU Report 62 [7], margins were suggested to account for variations in size, shape and position of the CTV in relation to anatomical reference points, perhaps as the result of the filling of the stomach or bladder or movement due to respiration. This volume was called the internal margin. To this was to be added a set-up margin to take into account all the uncertainties in planning, patient positioning and beam positioning. These concepts are useful in understanding the basis of the PTV margin.
In ICRU Report 62 [7], it was accepted that under some circumstances, these margins need not be added arithmetically, but rather in quadrature, for example where movements do not occur in the same direction on the same occasion. The report also acknowledged that under some circumstances the PTV might have to be reduced, and very occasionally the CTV reduced as well, in order to limit dose to an adjacent critical normal tissue. This is an important concept, especially in circumstances where higher radiotherapy doses are being attempted.
In its report of 2003 [8], the BIR showed how to calculate the PTV margin in more detail. It suggested abandoning the internal and set-up margins proposed in ICRU Report 62 and replacing these with a systematic error margin and a random error margin. The CTV plus the systematic error margin gives the systematic target volume (STV), and the addition of the random error margin gives the PTV. The distinction between systematic and random errors in the radiotherapy process is also very helpful. Broadly, systematic errors arise in treatment preparation, whereas random errors are attributable to errors in execution. This report shows how to deal with errors and uncertainties, and how to combine them. Typically, a larger margin is required for preparation errors than for execution errors since some blurring of the execution errors happens over a course of treatment. Although errors in setting up the patient are always 3D, it is extremely difficult to measure the 3D error. The BIR report provides a method in which the error in each dimension can be incorporated separately. It also contains a spreadsheet in which to calculate margins, and provides site-specific recommendations.
ORs
ORs are normal tissues whose radiation sensitivity influences treatment planning or the prescribed radiation dose. Both systematic and random errors apply to ORs just as much as to the CTV. In that case, a margin should be added to the OR, which is analogous to the PTV margin around the CTV, and generates the PRV.
However, adding a PRV around an OR will very substantially increase the volume of the normal tissue structures and may present dilemmas concerning the radiotherapy dose to the target. Fortunately, this is only needed in certain circumstances. It is helpful to create a PRV around an OR whose damage is especially dangerous, and particularly where loss of a small amount of normal tissue from radiation damage would produce a severe clinical manifestation. A good example of this is the spinal cord, a ’serial’ tissue in organisation (analogous to an electrical circuit), whose damage is catastrophic for the patient. Around the spinal cord it is advisable to add a PRV if doses which exceed the tolerance of the spinal cord are intended. It must be accepted that some interaction between the PTV and a PRV may be necessary, and may influence the prescribed radiation dose and dose distribution.
Radical and palliative radiotherapy
Concepts of conformal therapy have most often been applied to radical, curative radiotherapy. However, the concepts of conformal radiotherapy apply equally well to palliation, where avoidance of normal tissue side effects is intrinsic in the concept of good palliative care. Within the palliative setting, it may be reasonable to set a minimal CTV margin, or perhaps to use no CTV margin at all. Clearly, this is a clinical decision which is patient-specific.
Conclusion
The concepts of GTV, CTV and PTV have been enormously helpful in allowing radiation oncologists to develop treatment protocols. All of these volumes are crucially dependent on high quality imaging. Imaging to define the gross tumour is essential as the starting point for a radiotherapy treatment plan. Imaging of tumour spread, which has enormous potential for future development, is essential to contribute to the determination of the CTV. Further imaging of the surrounding anatomy is necessary to define critical normal structures. Finally, in assessing the margin required for a PTV, errors intrinsic in scanners, electronic transfer protocols, accuracy of co-registration of different imaging modalities, and the quality of the imaging are all factors to be considered. Attention to the technicality of planning is important, and deviation from optimum protocols has been shown to reduce local control and cure. For radiotherapy, there is no doubt that ‘the devil is in the detail’.
It should be obvious that radiotherapy planning is completely dependent on diagnostic imaging, and benefits from the close involvement of diagnostic radiologists and radiographers.
Key points
The definition of tumour and target volumes for radiotherapy is vital to the successful execution of radiotherapy.
Attention to the detail of radiotherapy planning actually improves outcomes.
Radiotherapy requires the best possible diagnostic imaging to define the location and extent of the tumour.
The GTV describes what can be seen, palpated or imaged.
The CTV contains the GTV plus a margin for sub-clinical disease spread which cannot be fully imaged.
The CTV must be adequately treated to achieve cure.
Delineation of the CTV is likely to benefit from future developments in imaging, especially towards the molecular level.
The PTV allows for uncertainties in planning or treatment delivery, and is designed to ensure that the radiotherapy dose is actually delivery to the CTV.
The PTV is a geometric concept designed to ensure adequate treatment of the CTV, and can therefore extend outside the patient in some circumstances.
Normal tissue must be considered in radiotherapy planning, and relevant structures are termed organs at risk (ORs).
References
- 1.Swedish Council on Technology Assessment in Health Care (SBU) Radiotherapy for cancer. Acta Oncol. 1996;35(Suppl 6):1–100. (Suppl 7): 1–152. [Google Scholar]
- 2.Suit H. Contributions of L.H. Gray to radiation physics, biology, and oncology. Int J Radiat Oncol Biol Phys. 2002;53(4):795–7. doi: 10.1016/s0360-3016(02)02852-3. [DOI] [PubMed] [Google Scholar]
- 3.Burnet NG, Wurm R, Nyman J, Peacock JH. Normal tissue radiosensitivity—how important is it? Clin Oncol. 1996;8:25–34. doi: 10.1016/s0936-6555(05)80035-4. [DOI] [PubMed] [Google Scholar]
- 4.Horiot JC, Le Fur R, N’Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomised trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25(4):229–30. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 5.Yarnold JR, Owen JR, Ashton A, et al. Fractionation sensitivity of change in breast appearance after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2002;64(Suppl 1):S25. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.ICRU. Prescribing, Recording and Reporting Photon Beam Therapy. Report 50. Bethesda, MD: International Commission on Radiation Units and Measurements, 1999
- 7.ICRU. Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). Report 62. Bethesda, MD: International Commission on Radiation Units and Measurements, 1999
- 8.Geometric Uncertainties in Radiotherapy—Defining the Planning Target Volume. British Institute of Radiology; 2003. [Google Scholar]