Abstract
Oxidants, including hydrogen peroxide (H2O2), have been recognized for years to mimic insulin action on glucose transport in adipose cells. Early studies also demonstrated the complementary finding that H2O2 was elaborated during treatment of cells with insulin, suggesting that cellular H2O2 generation was integral to insulin signaling. Recently, reactive oxygen species elicited by various hormones and growth factors have been shown to affect signal transduction pathways in various cell types. We recently reported that insulin-stimulated H2O2 modulates proximal and distal insulin signaling, at least in part through the oxidative inhibition of protein tyrosine phosphatases (PTPases) that negatively regulate the insulin action pathway. Nox4, a homologue in the family of NADPH oxidase catalytic subunits, was found to be prominently expressed in insulin-sensitive cells. By various molecular approaches, Nox4 was shown to mediate insulin-stimulated H2O2 generation and impact the insulin signaling cascade. Overexpression of Nox4 also significantly reversed the inhibition of insulin-stimulated receptor tyrosine phosphorylation by PTP1B, a widely expressed PTPase implicated in the negative regulation of insulin signaling, by inhibiting its catalytic activity. These recent studies have provided insight into Nox4 as a novel molecular link between insulin-stimulated reactive oxygen species and mechanisms involved in their modulation of insulin signal transduction.
REACTIVE OXYGEN SPECIES IN INSULIN SIGNALING
Although high levels of circulating reactive oxygen species have been well documented to play an important role in the pathogenesis of tissue damage in the complications of diabetes mellitus (9), senescence (23), and other related pathophysiological processes, it has been less appreciated that smaller amounts of superoxide and hydrogen peroxide (H2O2) are generated by insulin stimulation at the cellular level, and play a role in facilitating normal signal transduction by insulin, as well as a variety of other hormones and growth factors. In fact, the initial recognition that insulin can elicit reactive oxygen species in its target cells was made over 30 years ago. Recent work has begun to elucidate at a molecular level the cellular mechanisms of reactive oxygen species generation in response to insulin, involving the NADPH oxidase system, and some of the enzymatic targets regulated by reactive oxygen species, including protein tyrosine phosphatases (PTPases), and potentially other enzymes that are dependent on the reduced state of a critical thiol moiety for their catalytic activity.
OXIDANTS AS INSULIN MIMICKERS AND SECOND MESSENGERS
The potential involvement of oxidant species in insulin signaling was initially explored in the early 1970s, with the observation by Czech and colleagues that certain metal cations interacting with albumin could transfer electrons to a cellular target and enhance glucose utilization by adipocytes (14-16). Livingston and colleagues also contributed to these early observations in their studies on insulin mimickers, including polyamines, which were also found to act via the generation of H2O2 (45). Other groups also provided similar early observations in this area (50).
A few years later, it was also reported that the stimulation of glucose uptake in adipose cells by insulin was accompanied by sulfhydryl oxidation (51, 56, 57). As early as 1977, some of the enzymological characterization of this process was already being elucidated. Insulin was shown to activate a plasma membrane enzyme system with the properties of an NADPH oxidase, resulting in the downstream production of H2O2 (35, 52, 55, 57). These results initiated the development of a new regulatory mechanism for insulin signaling involving a plasma membrane oxidase stimulated by insulin that generates superoxide and H2O2 by the action of cellular superoxide dismutase. Both of these reactive oxygen species were postulated to have regulatory effects on the insulin action cascade.
TYROSINE PHOSPHORYLATION OF THE INSULIN RECEPTOR AND ACTIVATION OF DOWNSTREAM INSULIN SIGNALING
Understanding the molecular mechanisms of insulin signaling laid the foundation for determining the mechanism by which cellular reactive oxygen species might modulate the insulin action cascade (Fig. 1). The insulin receptor was identified as a ligand-activated tyrosine kinase (20, 70) that under-went autophosphorylation and catalyzed the tyrosine phosphorylation of its cellular substrate IRS proteins (31, 67). The activation of the intracellular tyrosyl-specific protein kinase domain of the insulin receptor is essential for virtually all of insulin’s growth-promoting and metabolic effects (63). Insulin binding elicits the rapid autophosphorylation of specific tyrosine residues as a cascade (24, 74). In the receptor kinase domain, two of the three regulatory tyrosyl residues become rapidly phosphorylated upon receptor activation, followed by phosphorylation of the third tyrosyl residue, which leads to full activation of the receptor kinase toward exogenous substrates. Thus, at the very least, the transition between the bis- and tris-phosphorylated forms of the receptor regulatory domain serves as a discrete molecular “switch” that determines the overall degree of insulin receptor kinase activation (30). This rapid reversibility of receptor phosphorylation is a key feature that helps to determine the level of activation of insulin signaling in the cell.
FIG. 1.
Potential sites of action for reactive oxygen species in the regulation of the insulin signaling pathway. This simplified scheme shows the major pathways of insulin signaling, involving activation of the receptor tyrosine kinase cascade leading initially to the tyrosine phosphorylation of the receptor and its cellular substrate IRS proteins and Shc (63). The steady-state level of tyrosine phosphorylation of these proteins is regulated by PTPases, a family of enzymes that constitute one of the key targets of regulation by reactive oxygen species in the cell. Additional thiol-sensitive regulatory proteins that strongly impact on the insulin action cascade include PTEN, PP2A, and MKP-1. See text for discussion and additional references. GSK3, glycogen synthase kinase 3; PDK, phospholipid-dependent kinase; PKC, protein kinase C.
The prototypic IRS protein, IRS-1, and the other members of the IRS family contain multiple tyrosyl residues that are efficiently phosphorylated by the receptor kinase (73). The IRS proteins act as adapter or “docking” scaffolds for the binding of a several downstream proteins that transmit the insulin signal by forming tight but noncovalent associations via their Src homology 2 (SH2) domains with the phosphotyrosyl side chains of IRS, including, for example, the p85 subunit of phosphatidylinositol (PI) 3′-kinase, the SH2/SH3 adapter proteins growth receptor bound protein 2 (Grb2) and Nck, and an intracellular PTPatase, SHP-2 (Fig. 1). Once bound, these proteins are activated by conformational changes and the subcellular compartmentalization resulting from this interaction (63). PI 3-kinase, activated by the docking of its p85 subunit to phosphorylated IRS-1, is linked to the activation of glucose transport, p70 S6 kinase activation, and nuclear DNA synthesis and gene expression. PI 3-kinase is also linked to activation of the protein kinase Akt (protein kinase B) and further downstream responses involving glucose transporter 4 (GLUT4) vesicle translocation and glucose transport activation. Binding of SHP-2 to IRS-1 activates its intrinsic PTPase activity, which is involved in mitogenic signaling. Grb2 links IRS-1 phosphorylation to the Ras pathway via SOS (son of sevenless protein), which stimulates p21ras activation. Alternatively, insulin-stimulated tyrosine phosphorylation of Shc enables it to bind Grb2, which can then also activate Ras. Downstream of Ras, insulin signaling is coupled to mitogen-activated protein (MAP) kinase kinase (MEK), which activates the MAP kinase pathway.
REVERSIBLE TYROSINE PHOSPHORYLATION IN THE REGULATION OF INSULIN SIGNALING
As purified insulin receptors retain their autophosphorylation state even after insulin is removed from the ligand binding site (29, 34), it has been recognized that cellular PTPase activity plays a pivotal role in the down-regulation of receptor kinase activity and the insulin action cascade. Given the multisite phosphorylation of various tyrosine residues on the insulin receptor and its substrate proteins, different PTPases, or mechanisms that regulate their interaction with specific phos-photyrosine sites on various IRSs, may play an important regulatory role in differentially affecting postreceptor insulin signaling (27).
CANDIDATE PTPASES FOR THE REGULATION OF INSULIN SIGNALING
The family of PTPase enzymes is extensive, and various homologues are involved in a variety of signal transduction pathways. In insulin signaling, evidence from a variety of laboratories has demonstrated a regulatory role for the intracellular enzyme protein tyrosine phosphatase 1B (PTP1B) (26, 68), and to a lesser extent other homologues, including the transmembrane receptor-type enzyme LAR (leukocyte antigen related) (27, 39, 59, 77), and more recently, the intracellular enzyme TC-PTP (T-cell phosphatase) (25). The considerable data implicating PTP1B as a negative regulator of the insulin action pathway have included manipulation of PTP1B expression, or expression of recombinant, inactive PTP1B constructs that demonstrate effects on insulin action, as well as a physical association between PTP1B and the autophos-phorylated insulin receptor (for recent reviews, see 26, 68). The most compelling evidence for PTP1B in insulin signaling stems from studies in two independently derived lines of transgenic mice showing that PTP1B is a key regulator of insulin action in vivo (21, 33). Although the PTP1B knockout mice had no obvious disease phenotype and normal fetal viability, they exhibited a heightened level of insulin sensitivity with enhanced glucose and insulin tolerance. The mechanism of improved insulin responsiveness was related to an enhancement of insulin receptor autophosphorylation that occurred in a tissue-specific fashion, with enhanced insulin responsiveness in skeletal muscle and liver that was relatively unchanged in adipose tissue.
PTPASES ARE THIOL-DEPENDENT ENZYMES REGULATED BY THE B>CELLULAR REDOX STATE
A key feature of PTPases in the regulation of the insulin action pathway is tied in with the recognition that these enzymes are regulated by alterations of the redox state of the PTPase catalytic center. As an enzyme family, the PTPases share a common catalytic motif, a conserved ∼230 amino acid domain that contains an 11-residue signature sequence consisting of (I/V)HCXAGXXR(S/T/G) (1, 27, 69). This sequence includes the cysteine residue required to catalyze the hydrolysis of protein-phosphotyrosine residues by the formation of a cysteinyl-phosphate intermediate (4, 18). The reactivity of the catalytic thiol residue is determined by hydrogen bonding in the three-dimensional structure of the enzyme that lowers its pKa and favors a relatively ionized state of the cysteinyl hydrogen (61).
The stepwise oxidation of the catalytic cysteine residue of PTPases by reactive oxygen species to more inert forms has been recognized to be a major source of their enzymatic regulation in vivo (17, 22, 43, 53) (Fig. 2). Initially, the catalytic cysteine thiol is oxidized to the sulfenic (-SOH) form, which is a reversible alteration, amenable to reduction by cellular enzymatic mechanisms or with reducing agents in vitro, which restores the catalytic thiol group (13, 19). Further sequential steps of oxidation, to sulfinic (-SO2H) and sulfonic (-SO3H) forms, can lead to irreversible PTPase inactivation (5). Additionally, PTPases can undergo disulfide conjugation in the cell with glutathione, forming an inactive glutathiolated form, which is also potentially reversible by specific cellular reductases (6). This general scheme appears to constitute a major regulatory mechanism for PTPases within the cellular environment and directly impacts on their effects on signal transduction.
FIG. 2.
Regulation of PTPase catalytic activity by oxidation, conjugation, and reduction of the catalytic cysteine residue.A key feature of PTPases in the regulation of signal transduction is the modulation of their activity by alterations of the oxidation-reduction state of the enzyme catalytic center. The catalytic cysteine of the PTPases is especially reactive because of the low pKa of the sulfhydryl that favors a relatively ionized state of the cysteinyl hydrogen (61), and stepwise oxidation of the catalytic cysteine residue of PTPases by reactive oxygen species to more inert forms is a major source of enzymatic regulation in vivo (17, 23, 53). Oxidation of the catalytic thiol to the sulfenic (—SOH) form is reversible. Higher order oxidation to sulfinic (—SO2H) and sulfonic (—SO3H) forms can lead to irreversible PTPase inactivation. Mildly oxidized PTPases can undergo disulfide conjugation in the cell with glutathione (6). A novel sulfenyl-amide derivative of PTP1B has been shown to undergo GSH conjugation or direct biochemical reduction with DTT (62, 72). Active enzyme can be regenerated from glutathiolated enzyme by cellular GSH reductases. Whereas the sulfonic-acid derivative of the active site cysteine is felt to be irreversible, the sulfinic-acid derivative may be reduced by a novel class of recently described sulfiredoxin enzymes (7) to a conjugated form that precedes the regeneration of the active enzyme. These mechanisms of PTPase regulation are an active area of current research. See text for further discussion and references.
Recent work has also identified additional novel means by which the activity of partially oxidized protein thiol side-chains can be regenerated (61). A novel class of ‘sulfiredoxin’ enzymes capable of reducing protein cysteine-sulfinic acid in yeast has been recognized (7). This sulfiredoxin reaction requires ATP and magnesium, and its mechanism involves activation by phosphorylation followed by a thiol-mediated reduction step. The rapid reduction of the sulfinic form of peroxiredoxin I to the catalytically active thiol form has also been demonstrated, consistent with a new type of cyclic modification by which the enzymatic function of these redox-sensitive proteins is regulated (75). It remains to be demonstrated whether PTPases oxidized to the sulfinic form are amenable to reduction and regeneration by this novel mechanism.
The sulfenic acid intermediate produced by mild oxidation of PTP1B has also recently been shown to be rapidly converted to a previously unknown sulfenyl-amide species, in which the sulfur atom of the catalytic cysteine is covalently linked to the main-chain nitrogen of an adjacent residue (62, 72). Oxidation of PTP1B to the sulfenyl-amide form is accompanied by large conformational changes in the catalytic site that inhibit substrate binding. This novel structure appears to be a stabilizing modification that protects the activesite cysteine from further irreversible oxidation to sulfonic acid and at the same time serves to regulate the enzyme by promoting its reversible reduction by thiols. A novel regulatory scheme has thus been developed whereby this intermediate can be reversed by thiol conjugation with glutathione or other thiol reactive groups in the cell (62, 72).
The intracellular circuits that regulate the catalytic activity of the PTP1B are consistent with studies demonstrating that the activity of PTP1B (and other PTPases) assayed in vitro can be dissociated from their tissue protein mass (8, 12). As the assay of PTPases from tissues and cell lysates is typically performed under conditions of strong biochemical reduction, the mild levels of enzyme oxidation that might occur in the intact cells can be overcome in vitro. Using a controlled anaerobic environment for enzyme assays (78), we reported that the endogenous PTPase activity in omental adipose tissue was more than two-fold higher than the activity in paired samples of subcutaneous adipose tissue (76). A substantial fraction of PTPase activity in human adipose tissue was also found to be present in a latent, oxidized form that can be reactivated to various degrees by biochemical reduction in vitro, confirming that reversible oxidation serves as an important means of PTPase regulation in vivo (78).
INSULIN RECEPTOR ACTIVATION AS A POTENTIAL SITE OF REDOX MODULATION
Novel evidence for redox modulation of insulin signaling was also reported in a series of articles by Schmid et al. (64, 65), who showed that antioxidant treatment of cells inhibits insulin responsiveness. Conversely, creating mildly oxidative intracellular conditions decreased the sulfhydryl content of the insulin receptor β-chain and enhanced the activation of the receptor by insulin. Kinetic analysis of the receptor kinase activation suggested that optimal insulin responsiveness involved a process of “redox priming” of the β-subunit. Further studies suggested that phosphocreatine in combination with H2O2 may serve as an alternative phosphate donor for the insulin receptor at a distinct binding site from ATP.
ADDITIONAL POTENTIAL REDOX TARGETS IN INSULIN-SENSITIVE CELLS
Several additional enzymes that regulate downstream components in the insulin signaling cascade are also potential targets of redox regulation (Fig. 1). For example, the serine protein phosphatase PP2A, which has been implicated in the negative regulation of Akt by dephosphorylation of Ser-473, has a redox-sensitive cysteine residue that is potentially susceptible to inhibition by H2O2 (28). The dual-specificity (tyr/ser) phosphatase MAP kinase phosphatase 1 (MKP-1), which attenuates insulin-stimulated MAP kinase activity (40), is also dependent on a reduced thiol for activity. As an immediate-early gene, MKP-1 mRNA expression is also increased in a variety of cell types by reactive oxygen species (32). The tumor suppressor PTEN (phosphatase and tensin homologue), which dephosphorylates the 3′-phosphate of inositol phospholipids generated by PI 3′-kinase, can modulate downstream signaling by insulin and a number of other growth factors (58). PTEN is inactivated by exposure to oxidant molecules, which cause a disulfide linkage between two essential cysteine residues in its active site (44). Subsequent reduction of H2O2-oxidized PTEN in cells appears to be mediated predominantly by thioredoxin, suggesting that this regulatory system provides a novel means of controlling the accumulation of 3′-phosphorylated phosphoinositides in the cell (44).
ROLE OF INSULIN IN THE GENERATION OF CELLULAR REACTIVE OXYGEN SPECIES
In recent studies, we have attempted to link the observations that a “burst” of intracellular reactive oxygen species resulting from cellular insulin stimulation may be associated with reversible oxidative inhibition of cellular PTPase activity (and potentially other cellular targets) that could modulate insulin signaling. Consistent with the prior work in this area cited above, we demonstrated that insulin stimulation generated a cellular oxidant signal in 3T3-L1 adipocytes loaded with a redox indicator dye based on 2′,7′-dichlorodihydrofluorescein (DCF) that is trapped intracellularly after cleavage by cellular esterases [5(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate] (47). Following stimulation of 3T3-L1 adipocytes with 100 nM insulin, a strong oxidant signal was detected by DCF fluorescence within 1 min, peaked at 5 min, and began to dissipate by 10 min (Fig. 3). The oxidant generated was shown to be H2O2, because preincubation of the cells with catalase eradicated the fluorescent signal, although it is also likely that the cellular H2O2 is derived from superoxide by dismutation in the cell. A doseresponse study using insulin exposure for 5 min showed that H2O2 production was detectable between 0.1 and 1 nM and was maximal at 10 nM. Similar results were observed in human HepG2 hepatoma cells, with an insulin-stimulated H2O2 signal evident at 1 min that increased through 10 min of incubation and was also completely blocked by catalase preincubation.
FIG. 3.

Insulin-stimulated production of H2O2 in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were serum-starved overnight prior to stimulation with insulin as shown. Intracellular H2O2 production was detected by preloading the cells with DCF (Molecular Probes) and detecting the fluorescent signal in situ by confocal microscopy. (A) Insulin time course performed using 100 nM insulin. (B) Insulin dose response performed with insulin stimulation for 5 min [modified from Mahadev et al. (47)].
EFFECTS OF INSULIN-STIMULATED REACTIVE OXYGEN SPECIES ON INSULIN SIGNALING
The insulin-stimulated oxidant signal significantly enhanced the level of tyrosine phosphorylation of the insulin receptor and IRS proteins in 3T3-L1 adipocytes (47). Catalase treatment had no effect on basal tyrosine phosphorylation, but reduced the insulin-stimulated autophosphorylation of the insulin receptor and the IRS proteins by ∼48% and ∼43%, respectively. These data suggested that the insulin-stimulated oxidant signal impacts early events in insulin action, potentially by the oxidative inhibition of negatively regulatory PTPases.
Looking into more distal signal interactions with the cellular oxidant-generating system, we used diphenyleneiodonium (DPI), an inhibitor of cellular NADPH oxidase activity, which blocked the insulin-stimulated cellular production of H2O2. Cellular treatment with DPI completely inhibited the activation of PI 3′-kinase activity by insulin and reduced the insulin-induced activation of the serine kinase Akt by up to 49% (46). The H2O2-induced activation of Akt was entirely mediated by upstream stimulation of PI 3′-kinase activity, because treatment of 3T3-L1 adipocytes with the PI 3′-kinase inhibitors wortmannin or LY294002 completely blocked the subsequent activation of Akt by exogenous H2O2. Interestingly, the effects we observed of H2O2 on downstream insulin signaling in the adipocyte model were similar to observations in other cell types, including fibroblasts, embryonic kidney cells, and vascular smooth muscle cells, where activation of Akt by H2O2 is also abrogated by inhibition of PI 3′-kinase (66, 71). These important findings suggest that cellular PI 3′-kinase is a critical upstream mediator of Akt activation by oxidative molecules in a variety of signaling pathways, including insulin action.
Preventing oxidant generation with DPI also blocked insulin-stimulated glucose uptake and GLUT4 translocation to the plasma membrane, providing further evidence for an oxidant signal in the regulation of the distal insulin-signaling cascade (46). Insulin stimulated a mean 3.7-fold increase in glucose uptake in 3T3-L1 adipocytes that was completely abrogated by prior incubation of the 3T3-L1 adipocytes with DPI. Similar results were obtained using a plasma membrane sheets assay, which assesses GLUT4 translocation to the plasma membrane.
ROLE OF INSULIN-STIMULATED H2O2 IN MAP KINASE ACTIVATION
Although blocking the insulin-induced rise in H2O2 with DPI strongly attenuated the activation of the PI 3′-kinase pathway by insulin, this treatment paradoxically led to an increase in the activation of p42/p44 MAP kinase (49). DPI inhibited insulin-stimulated receptor and IRS-1/2 tyrosine phosphorylation, and also reduced the association of Grb2 with IRS-1, suggesting that the effect of DPI on MAP kinase activation occurred downstream of the insulin receptor and IRS proteins. As DPI enhanced basal Grb2-SOS binding and reduced the effect of insulin to potentiate the dissociation of the Grb2-SOS complex, the effect of DPI was apparently mediated upstream of Raf-1 (Fig. 1). These studies revealed that the insulin-stimulated oxidant signal may differentially affect the two major downstream components of the insulin signaling pathway, involving PI 3′-kinase and MAP kinase.
EFFECTS OF INSULIN-STIMULATED REACTIVE OXYGEN SPECIES ON PTPASE ACTIVITY
Considering the sensitivity of cellular PTPases to oxidizing molecules, and their relevance to the regulation of insulin signal transduction, we explored how insulin stimulation leads to alterations in total PTPase activity, as well as specifically of PTP1B, a major candidate PTPase for the regulation of insulin action (26). We used a novel approach that involves sample handling and analysis under anaerobic conditions to preserve the endogenous activity of PTPases as isolated from the cultured cells and avoid oxidation and artifactual enzyme inhibition that occurs on exposure to air (78). Treatment of HepG2 cells with 100 nM insulin for 5 min resulted in a 32-52% reduction in overall PTPase activity in the cell homogenate, the cytosol, and the solubilized particulate fraction. Biochemical reduction of the enzyme samples with dithiothreitol (DTT) prior to PTPase assay had no significant effect on the control samples prior to insulin treatment, but fully restored the reduced PTPase activity of the insulin-treated samples, indicating that they had been reversibly oxidized and inactivated by insulin exposure. A similar but more striking effect was observed using 3T3-L1 adipocytes, where insulin treatment caused a 62% drop in PTPase activity in the cell lysate, which was restored to within control levels by treatment of samples with DTT prior to enzyme assay (47).
To determine the role of insulin-induced H2O2 in the oxidative inhibition of cellular PTPase activity, cells were preincubated with catalase prior to insulin stimulation and PTPase assay (47). Catalase had no significant effect on the basal level of PTPase activity. However, the presence of catalase blocked the reduction of PTPase activity in the 3T3-L1 adipocyte cell lysate induced by insulin to a level that was not significantly different from the control samples. This important finding indicated that H2O2 mediated the oxidative inhibition of cellular PTPase activity (47).
Insulin-stimulated generation of H2O2 also affected the specific activity of PTP1B as isolated from intact cells (47). We immunoprecipitated PTP1B from snap-frozen HepG2 cell lysates under anaerobic conditions and assayed its activity within the anaerobic chamber. Following insulin treatment for 2-5 min, the activity of immunoprecipitated PTP1B was reduced to 46% and 29% of control, respectively. In the continued presence of insulin, this effect was sustained for at least 10 min. In 3T3-L1 adipocytes, insulin treatment also potently reduced the activity of immunoprecipitated PTP1B to 12% of control, which was reversible to 72% of control by preincubation of the immunoprecipitated enzyme with DTT prior to PTPase assay (47). Catalase pretreatment also abolished the insulin-induced inhibition of PTP1B. These findings provided support for the hypothesis that the insulin-stimulated H2O2 signal enhances the insulin-stimulated cascade of tyrosine phosphorylation by oxidative inactivation of PTP1B and possibly other tyrosine phosphatases.
EVIDENCE THAT THE NADPH OXIDASE HOMOLOGUE NOX4 MEDIATES INSULIN-STIMULATED GENERATION OF H2O2
The large amount of cellular reactive oxygen species elaborated by hematopoietic phagocytic cells in the process of host defense in bactericidal killing is generated by an NADPH oxidase complex that has been extensively characterized (2). The phagocytic oxidase complex consists of six subunits, including two plasma membrane-associated proteins, gp91phox (the catalytic subunit, now also designated as Nox2) and p22phox, which comprise flavocytochrome b558, and four cytosolic factors, p47phox, p67phox, p40phox, and rac (2). With the recognition that low levels of oxidant production are coupled to cellular growth factor and insulin stimulation (36, 37, 46, 47, 53, 60), Lambeth and colleagues recently identified a small family of five homologous Nox (NADPH oxidase) enzymes that are variably expressed in non-hematopoietic tissues (11, 41).
To determine which of the five members of this family may have a role in the insulin action pathway, we used RT-PCR, combined with northern and western blot analysis, and found that Nox4, a homologue of gp91phox, is highly expressed in insulin-sensitive adipose and liver cells (48). We then explored the potential role of Nox4 in the generation of H2O2 by insulin stimulation and in the regulation of cellular insulin action using overexpression of wild-type Nox4, as well as two dominant-negative constructs lacking binding domains for NADPH or FAD/NADPH by adenovirus-mediated gene delivery in differentiated 3T3-L1 adipocytes. Overexpression of Nox4 increased insulin-stimulated H2O2 generation by 21%, as assessed by DCF fluorescence. Importantly, both deletion constructs acted in a dominant-negative fashion and markedly attenuated insulin-stimulated H2O2 generation by 75%. Insulin-stimulated tyrosine phosphorylation of the insulin receptor and IRS proteins was not significantly changed in cells overexpressing wild-type Nox4, but was decreased by as much as 56% and 58%, respectively, in cells expressing the dominant-negative Nox4 deletion constructs (48).
Complementary studies with an RNAi approach to reduce the protein mass of Nox4 by up to ∼50% in 3T3-L1 adipocytes decreased insulin-stimulated receptor tyrosyl phosphorylation by as much as 64% and decreased Akt phosphorylation up to 48% compared with cells transfected with a scrambled control small inhibitory RNA (48). Overall, these data provide strong evidence that Nox4 plays an important role in the regulation of the insulin signaling pathway.
ROLE OF NOX4 IN THE MODULATION OF INSULIN RECEPTOR TYROSINE PHOSPHORYLATION BY PTP1B
To determine if Nox4 influenced the effect of PTP1B on insulin signal transduction, we cotransduced two recombinant adenoviruses to increase the level of expression of both PTP1B and wild-type Nox4 or the dominant-negative FAD-NADPH Nox4 deletion construct (48). As expected, overexpression of PTP1B alone reduced the insulin-stimulated autophosphorylation of the insulin receptor by 67%. Coexpression of Nox4 along with PTP1B significantly diminished the effect of PTP1B alone on insulin receptor autophosphorylation by 46%. This effect was due to the catalytic activity of Nox4, because expression of the FAD-NADPH deletion construct had no effect on the inhibition of insulin-stimulated receptor autophosphorylation induced by PTP1B overexpression. These findings suggest that the effect of PTP1B to inhibit insulin signal transduction can be blocked by the generation of reactive oxygen species by Nox4.
MECHANISM OF INSULIN-INDUCED H2O2
Cellular H2O2 generated by platelet-derived growth factor (PDGF) stimulation of HepG2 cells expressing recombinant PDGF receptors was shown to require PI 3′-kinase activation (3). In contrast, we found that H2O2 production in response to insulin in 3T3-L1 cells was not sensitive to PI 3-kinase inhibitors, suggesting that this process may bypass a key postreceptor regulatory pathway for insulin’s metabolic effects (46). Detailed studies need to be done, however, to characterize how activation of the insulin receptor may be coupled to the NADPH oxidase complex.
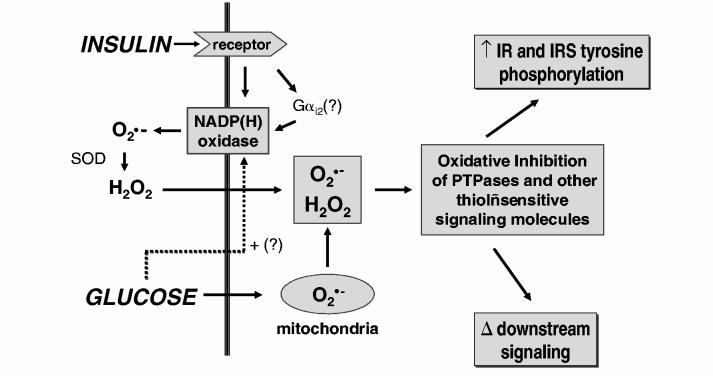
Small GTP-binding proteins, including Rac-1, have been shown to be involved in the activation of NADPH oxidase in response to growth factor and agonist stimulation (3, 42). This observation may be relevant to work by Krieger-Brauer and colleagues showing that the elaboration of cellular H2O2 during physiological insulin signal transduction is generated by a novel plasma membrane-bound Mn2+-dependent NADPH oxidase that is coupled to Gαi2 (Fig. 4) (35, 38). Other recent work has provided further evidence in several in vivo models that Gαi2 is linked to insulin action. For example, Gαi2 deficiency in a transgenic mouse model produces insulin resistance and impaired glucose tolerance (54); conversely, conditional expression of a constitutively active mutant of Gαi2 in insulin-sensitive tissues mimics insulin action in vivo with enhanced glucose tolerance and activation of adipocyte GLUT4 recruitment, hexose transport, and glycogen synthase (10). These data support the hypothesis that Gαi2 plays a permissive role for insulin signaling, possibly involving a mechanism that couples insulin signaling to the elaboration of reactive oxygen species, although this has not yet been directly evaluated.
FIG. 4.
Potential mechanisms and regulation of insulin-stimulated reactive oxygen species in insulin target cells. The generation of cellular reactive oxygen species [superoxide (), H2O2] to insulin is coupled to a plasma membrane NADPH oxidase mechanism that we have found to involve the recently described catalytic subunit homologue, Nox4 (48). Superoxide generated by the NADPH oxidase system is potentially converted to H2O2 species can play a role in modifying the catalytic activity of thiol-dependent regulatory enzymes in the cell, which can then alter by superoxide dismutase (SOD). Both of these reactive oxygen both proximal and distal insulin action (Fig. 1). The generation of reactive oxygen species by insulin may be enhanced by high glucose conditions, which increase mitochondrial superoxide production (9) and may also activate the NADPH oxidase system. Gαi2 may also modulate the activity of the insulin-sensitive NADPH oxidase pathway (35, 38). See text for further discussion. IR, insulin receptor.
HIGH GLUCOSE INCUBATION CONDITIONS MODULATE THE INSULIN-STIMULATED PRODUCTION OF H2O2
Finally, we have recently found that the high glucose conditions characteristic of diabetes mellitus and known to be associated with increased cellular reactive oxygen species (9) can potentiate the cellular H2O2 signal elicited by insulin (Fig. 4) (unpublished observations). 3T3-L1 adipocytes maintained in 25 mM glucose showed a threefold increase in basal cellular H2O2 (assessed by intracellular DCF fluorescence), compared with control cells (5 mM glucose). Following stimulation with 1 nM insulin for 5 min, intracellular H2O2 was increased 5.4-fold in 25 mM glucose compared with control conditions. Interestingly, at 100 nM insulin stimulation, the level of DCF fluorescence was similar under both glucose conditions, suggesting that high glucose increases the sensitivity of the oxidant signal to insulin stimulation. High glucose conditions also potentiated the inhibition of total cellular PTPase activity, as well as the endogenous activity of PTP1B, assayed under an inert atmosphere, consistent with enhanced inhibition of thiol-sensitive PTPases by increased cellular reactive oxygen species. Further work will help explore whether these cellular effects of high glucose significantly impact on insulin signal transduction pathways in vivo.
PERSPECTIVE
After three decades, the initial recognition that insulin elicits an oxidant signal in its target cells has achieved new relevance to insulin signaling. The detailed characterization of the role of reactive oxygen species in signal transduction by a variety of hormones and growth factors is currently an area of intense interest. Ongoing research is involved in characterizing the cellular targets that are susceptible to reversible and irreversible oxidation by superoxide and H2O2 elicited by various ligands, and the consequences of these reactions. The mechanism of coupling and regulation of the NADPH oxidase system to receptors for growth factors and insulin is also an area of keen interest. Further work in this area will help define the regulation of the cycles of oxidation and reduction of cellular PTPases and other regulatory enzymes that serve an important function in modulating enzymatic steps in the signal transduction cascade for insulin and other growth factors.
ACKNOWLEDGMENTS
We would like to thank J.N. Livingston for initiating our interest in this area many years ago. Also, we greatly appreciate the collaboration of Guangjie Cheng, Rebecca Arnold, and J. David Lambeth in our pursuit of the NADPH oxidases coupled to the insulin signaling cascade. The work from Dr. Goldstein’s laboratory was supported by NIH grant DK 43396.
Footnotes
ABBREVIATIONS
- Akt
- protein kinase B
- DCF
- 2′,7′-dichlorodihydrofluorescein
- DPI
- diphenyleneiodonium
- DTT
- dithiothreitol
- GLUT4
- glucose transporter 4
- Grb2
- growth receptor bound protein 2
- H2O2
- hydrogen peroxide
- IRS
- insulin receptor substrate
- MAP
- mitogen-activated protein
- MKP-1
- MAP kinase phosphatase 1
- Nox
- NADPH oxidase
- PDGF
- platelet-derived growth factor
- PI
- phosphatidylinositol
- PTEN
- phosphatase and tensin homologue
- PTPase
- protein tyrosine phosphatase
- PTP1B
- protein tyrosine phosphatase 1B
- SH2
- Src homology domain 2
- SOS
- son of sevenless protein.
REFERENCES
- 1.Andersen JN, Mortensen OH, Peters GH, Drake PG, Iversen LF, Olsen OH, Jansen PG, Andersen HS, Tonks NK, Moller NP. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 3.Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- 4.Barford D, Das AK, Egloff MP. The structure and mechanism of protein phosphatases—insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. doi: 10.1146/annurev.biophys.27.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 6.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Bio-chemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 7.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 8.Bleyle LA, Peng Y, Ellis C, Mooney RA. Dissociation of PTPase levels from their modulation of insulin receptor signal transduction. Cell Signal. 1999;11:719–725. doi: 10.1016/s0898-6568(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 10.Chen JF, Guo JH, Moxham CM, Wang HY, Malbon CC. Conditional, tissue-specific expression of Q205L G alpha i2 in vivo mimics insulin action. J Mol Med. 1997;75:283–289. doi: 10.1007/s001090050113. [DOI] [PubMed] [Google Scholar]
- 11.Cheng G, Cao Z, Xu X, Van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 12.Cheung A, Kusari J, Jansen D, Bandyopadhyay D, Kusari A, Bryer-Ash M. Marked impairment of protein tyrosine phosphatase 1B activity in adipose tissue of obese subjects with and without type 2 diabetes mellitus. J Lab Clin Med. 1999;134:115–123. doi: 10.1016/s0022-2143(99)90115-4. [DOI] [PubMed] [Google Scholar]
- 13.Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ, Charrier V, Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 14.Czech MP, Fain JN. Cu++-dependent thiol stimulation of glucose metabolism in white fat cells. J Biol Chem. 1972;247:6218–6223. [PubMed] [Google Scholar]
- 15.Czech MP, Lawrence JC, Jr, Lynn WS. Evidence for electron transfer reactions involved in the Cu2+-dependent thiol activation of fat cell glucose utilization. J Biol Chem. 1974;249:1001–1006. [PubMed] [Google Scholar]
- 16.Czech MP, Lawrence JC, Jr, Lynn WS. Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc Natl Acad Sci U S A. 1974;71:4173–4177. doi: 10.1073/pnas.71.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGnore JP, Konig S, Barrett WC, Chock PB, Fales HM. Identification of the oxidation states of the active site cysteine in a recombinant protein tyrosine phosphatase by electrospray mass spectrometry using on-line desalting. Rapid Commun Mass Spectrom. 1998;12:1457–1462. doi: 10.1002/(SICI)1097-0231(19981030)12:20<1457::AID-RCM346>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Denu JM, Dixon JE. Protein tyrosine phosphatases— mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 19.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide—evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 20.Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, Ou J-H, Masiarz F, Kan YW, Goldfine ID, Roth RA, Rutter WJ. Human insulin receptor cDNA: the structural basis for hormone activated transmembrane signalling. Cell. 1985;40:747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- 21.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 22.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 23.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Riveros JR, Sibley E, Kastelic T, Lane MD. Substrate phosphorylation catalyzed by the insulin receptor tyrosine kinase. Kinetic correlation to autophosphorylation of specific sites in the beta subunit. J Biol Chem. 1989;264:21557–21572. [PubMed] [Google Scholar]
- 25.Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, Puryer MA, Meng TC, Tonks NK, Tiganis T. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol Cell Biol. 2003;23:2096–2108. doi: 10.1128/MCB.23.6.2096-2108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein BJ. Protein-tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:265–275. doi: 10.2174/1568008013341163. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein BJ. Protein-tyrosine phosphatases and the regulation of insulin action. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus: A Fundamental and Clinical Text. Vol. 3. Lippincott; Philadelphia: 2003. pp. 255–268. [Google Scholar]
- 28.Guy GR, Cairns J, Ng SB, Tan YH. Inactivation of a redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993;268:2141–2148. [PubMed] [Google Scholar]
- 29.Haring HU, Kasuga M, White MF, Crettaz M, Kahn CR. Phosphorylation and dephosphorylation of the insulin receptor: evidence against an intrinsic phosphatase activity. Biochemistry. 1984;23:3298–3306. doi: 10.1021/bi00309a028. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasuga M, Zick Y, Blithe DL, Crettaz M, Kahn CR. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982;298:667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- 32.Keyse SM. Protein phosphatases and the regulation of MAP kinase activity. Semin Cell Dev Biol. 1998;9:143–152. doi: 10.1006/scdb.1997.0219. [DOI] [PubMed] [Google Scholar]
- 33.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalski A, Gazzano H, Fehlmann M, Van Obberghen E. Dephosphorylation of the hepatic insulin receptor: absence of intrinsic phosphatase activity in purified receptors. Biochem Biophys Res Commun. 1983;117:885–893. doi: 10.1016/0006-291x(83)91679-0. [DOI] [PubMed] [Google Scholar]
- 35.Krieger-Brauer HI, Kather H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992;89:1006–1013. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieger-Brauer HI, Kather H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem J. 1995;307:549–556. doi: 10.1042/bj3070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger-Brauer HI, Kather H. The stimulus-sensitive H2O2-generating system present in human fat-cell plasma membranes is multireceptor-linked and under antagonistic control by hormones and cytokines. Biochem J. 1995;307:543–548. doi: 10.1042/bj3070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem. 1997;272:10135–10143. doi: 10.1074/jbc.272.15.10135. [DOI] [PubMed] [Google Scholar]
- 39.Kulas DT, Zhang WR, Goldstein BJ, Furlanetto RW, Mooney RA. Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J Biol Chem. 1995;270:2435–2438. doi: 10.1074/jbc.270.6.2435. [DOI] [PubMed] [Google Scholar]
- 40.Kusari AB, Byon J, Bandyopadhyay D, Kenner KA, Kusari J. Insulin-induced mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) attenuates insulin-stimulated MAP kinase activity: a mechanism for the feed-back inhibition of insulin signaling. Mol Endocrinol. 1997;11:1532–1543. doi: 10.1210/mend.11.10.9998. [DOI] [PubMed] [Google Scholar]
- 41.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Lassegue B, Clempus RE. Vascular NADPH oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 43.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 44.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 45.Livingston JN, Gurny PA, Lockwood DH. Insulin-like effects of polyamines in fat cells. Mediation by H2O2 formation. J Biol Chem. 1977;252:560–562. [PubMed] [Google Scholar]
- 46.Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JTR, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 47.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 48.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng GJ, Lambeth JD, Goldstein BJ. The NADPH oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahadev K, Wu X, Motoshima H, Goldstein BJ. Integration of multiple downstream signals determines the net effect of insulin on MAP kinase vs. PI 3′-kinase activation: Potential role of insulin-stimulated H2O2. Cell Signal. 2004;16:323–331. doi: 10.1016/j.cellsig.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 50.May JM. The insulin-like effects of low molecular weight thiols: role of trace metal contamination of commercial thiols. Horm Metab Res. 1980;12:587–590. doi: 10.1055/s-2007-999206. [DOI] [PubMed] [Google Scholar]
- 51.May JM, de Haen C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem. 1979;254:9017–9021. [PubMed] [Google Scholar]
- 52.May JM, de Haen C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 53.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 54.Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit G ialpha2. Nature. 1996;379:840–844. doi: 10.1038/379840a0. [DOI] [PubMed] [Google Scholar]
- 55.Mukherjee SP, Lynn WS. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone’s effects on adenylate cyclase and the hexose monophosphate shunt. Arch Biochem Biophys. 1977;184:69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- 56.Mukherjee SP, Lynn WS. Role of cellular redox state and glutathione in adenylate cyclase activity in rat adipocytes. Biochim Biophys Acta. 1979;568:224–233. doi: 10.1016/0005-2744(79)90289-4. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 58.Nakashima N, Sharma PM, Imamura T, Bookstein R, Olefsky JM. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J Biol Chem. 2000;275:12889–12895. doi: 10.1074/jbc.275.17.12889. [DOI] [PubMed] [Google Scholar]
- 59.Ren JM, Li PM, Zhang WR, Sweet LJ, Cline G, Shulman GI, Livingston JN, Goldstein BJ. Transgenic mice deficient in the LAR protein-tyrosine phosphatase exhibit profound defects in glucose homeostasis. Diabetes. 1998;47:493–497. doi: 10.2337/diabetes.47.3.493. [DOI] [PubMed] [Google Scholar]
- 60.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. www.stke.org/cgi/content/full/OC_sigtrans. Sci STKE 2000. 2000:E1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 61.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 62.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 63.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 64.Schmid E, Elbenna J, Galter D, Klein G, Droge W. Redox priming of the insulin receptor beta-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB J. 1998;12:863–870. doi: 10.1096/fasebj.12.10.863. [DOI] [PubMed] [Google Scholar]
- 65.Schmid E, Hotz-Wagenblatt A, Hack V, Droge W. Phosphorylation of the insulin receptor kinase by phosphocreatine in combination with hydrogen peroxide: the structural basis of redox priming. FASEB J. 1999;13:1491–1500. doi: 10.1096/fasebj.13.12.1491. [DOI] [PubMed] [Google Scholar]
- 66.Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun XJ, Rothenberg PL, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 68.Tonks NK. PTP1B: from the sidelines to the front lines. FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- 69.Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–195. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 70.Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, Gray A, Coussens L, Liao Y-C, Tsubokawa M, Mason A, Seeburg PH, Grunfeld C, Rosen OM, Ramachandran J. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 71.Ushio-Fukai M, Alexander RW, Akers M, Yin QQ, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 72.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 73.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 74.White MF, Shoelson SE, Keutmann H, Kahn CR. A cascade of tyrosine autophosphorylation in the β-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988;263:2969–2980. [PubMed] [Google Scholar]
- 75.Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 76.Wu X, Hoffstedt J, Deeb W, Singh R, Sedkova N, Zilbering A, Zhu L, Park PK, Arner P, Goldstein BJ. Depot-specific variation in protein-tyrosine phosphatase activities in human omental and subcutaneous adipose tissue: a potential contribution to differential insulin sensitivity. J Clin Endocrinol Metab. 2001;86:5973–5980. doi: 10.1210/jcem.86.12.8109. [DOI] [PubMed] [Google Scholar]
- 77.Zhang WR, Li PM, Oswald MA, Goldstein BJ. Modulation of insulin signal transduction by eutopic overexpression of the receptor-type protein-tyrosine phosphatase LAR. Mol Endocrinol. 1996;10:575–584. doi: 10.1210/mend.10.5.8732688. [DOI] [PubMed] [Google Scholar]
- 78.Zhu L, Zilbering A, Wu X, Mahadev K, Joseph JI, Jabbour S, Deeb W, Goldstein BJ. Use of an anaerobic environment to preserve the endogenous activity of protein-tyrosine phosphatases isolated from intact cells. FASEB J. 2001;15:1637–1639. doi: 10.1096/fj.00-0795fje. [DOI] [PubMed] [Google Scholar]