Abstract
The periodontal pathogen Actinobacillus actinomycetemcomitans produces cytolethal distending toxin (CDT), a complex multicomponent toxin that arrests the growth of many types of eukaryotic cell. The kinetics of the effects of CDT-containing extracts, from an invasive strain of this bacterium, were examined on epithelial-like cells routinely used in invasion studies. Both KB and HEp-2 cells were exquisitely sensitive to the effects of the CDT with TD50 of 30 and 300 pg of total bacterial protein, respectively. Initial cell morphology changes were relatively rapid, occurring within the first 13 h of exposure. CDT-treated KB cells increased in size to 4–5 times the size of untreated controls. Cytotoxicity was irreversible when attached cells were incubated, for a minimum of 120 min, with nanogram quantities of CDT-containing extract. As cultures aged, the cells became more resistant to the effects of the CDT-containing extracts. These findings have important implications for understanding the ability of A. actinomycetemcomitans to invade and multiply in epithelial cells.
Keywords: Actinobacillus actinomycetemcomitans, cytolethal distending toxin, invasion, KB cells, Hep-2 cells
One of the unique properties of the oral gram-negative bacterium Actinobacillus actinomycetemcomitans is the production of several complex multigene toxins not found in other periodontal pathogens. The first multigene toxin system to be characterized in this bacterium was a leukotoxin (14). The leukotoxin has a relatively narrow host range affecting primarily neutrophils and monocytes in the human host. A second, more recently discovered, multicomponent cytotoxin in A. actinomycetemcomitans is the cytolethal distending toxin (CDT) (17, 28, 29).
The cdt locus in A. actinomycetemcomitans is comprised of three genes (cdtA–C) which appear to form a polycistronic operon (17, 27). The gene products for cdtA, cdtB and cdtC are approximately 27, 30 and 20 kDa, respectively, and expression of all three genes is required for cytotoxicity (17). Genetic homologs of the A. actinomycetemcomitans cdt genes are present in some strains of Escherichia coli (23, 26) and Shigella dysentarie (21), Campylobacter jejuni (24), Haemophilus ducreyi (7) and Helicobacter hepaticus (31). All of these bacterial species are considered to be pathogenic for humans.
Unlike the leukotoxin, exposure to the CDT is lethal for a wide variety of eukaryotic cells due to a generalized DNA-damaging activity (12, 15) and inhibition of the activation of a Cdc2––cyclin-B1 complex that is required to trigger mitosis in normal cells (6, 9, 20, 22, 25). HeLa, Hep-2 and Vero cells (13), Caco-2 cells (human colon carcinoma cell line) (30), HaCat cells (human keratinocyte cell line) (8), CD4+ and CD8+ T cells (28) and Henle-407 cells (intestinal epithelial cells) (15) exhibit the characteristic cell cycle arrest at the G2 phase when exposed to the CDT. It has been suggested that rapidly proliferating cells are the most sensitive to the activities of this toxin (5).
In theory, epithelial cells would be particularly sensitive to the CDT because they continuously proliferate and differentiate as they migrate from deeper layers toward the epithelial surface (5). KB and HEp-2 cells are the epithelial-like cell lines most commonly used in studies of the biological activities of oral pathogens. For example, several investigations have shown that select strains of A. actinomycetemcomitans have the ability to invade KB (16, 19) and HEp-2 cells (3) in culture. The invasion process consists of receptor-mediated endocytosis, escape from the vacuole, intracellular multiplication and intracellular and intercellular spread (18). The bacteria can multiply intracellularly in both types of cell. Therefore, it would appear that the potential for expression of the CDT by the internalized bacteria has important implications in invasion, colonization, and perhaps exploitation of the intracellular environment for replication. In this report we describe the kinetics of the response of KB and HEp-2 cells to the CDT produced by a clinically relevant invasive strain, A. actinomycetemcomitans UP54 (16). The effects of CDT-containing bacterial extracts from UP54 were compared to those of non-invasive CDT-producing strains and a naturally occurring CDT-negative strain obtained from the same geographically homologous subject population.
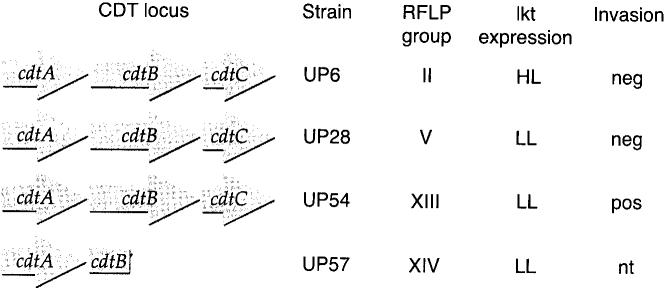
The A. actinomycetemcomitans strains originated from a collection of 900 isolates obtained from a regional population of families with localized aggressive periodontitis (11). The general properties of the strains used in this study are shown in Fig. 1. Strain UP54 was by far the most invasive strain among representatives of 14 different restriction fragment length polymorphism (RFLP) groups from the localized aggressive periodontitis family collection (16). This strain contained a complete cdt gene locus, as confirmed by restriction endonuclease mapping and DNA hybridization (17). Two other non-invasive CDT-producing strains (UP6 and UP28) and a CDT-negative strain (UP57) were employed as controls. Previous studies using Chinese hamster ovary cells confirmed that sonic extracts of UP54, UP6 and UP28 made an active toxin as measured by the characteristic distention and growth arrest characteristic of the CDT (17). Chinese hamster ovary cells grew normally when exposed to sonic extracts of strain UP57.
Fig. 1.
Properties of the A. actinomycetemcomitans strains used in this study. Restriction fragment length polymorphism (RFLP) group designations are defined in reference 10. Leukotoxin data are from reference 4. HL, high-level leukotoxin producer; LL, low-level leukotoxin producer; nt, not tested. Invasion data are from reference 16, except for strain UP57 which was unpublished.
All of the strains were previously subcultured, on TSB-YE medium (30 g tryptic soy broth, 3 g yeast extract and 10.5 g agar per litre), an average of 15 times to enhance conversion from a rough to the smooth phenotype required for invasion experiments (16). This conversion was necessary since homogenous cell suspensions could not be obtained with colonies of A. actinomycetemcomitans that have a rough phenotype. The smooth variants were grown on TSB-YE medium for 4 days for the kinetic experiments. The bacteria were washed twice with phosphate-buffered saline (pH 7.5), suspended in 5.0 ml α-minimal essential medium (αMEM) without serum and lysed by sonication for 3 min at 4°C (80% maximum speed at the high setting) using a Biosonik IV sonicator (Bronwill, Rochester, NY). Sonicates were centrifuged at 12 100g for 10 min to remove unbroken cells and insoluble cell debris. The supernatant fluids were sterilized by passage through 0.22-μm filters (Millipore, Bedford, MA) and stored in 1-ml aliquots at −70°C. Sonic extracts were used for cytotoxicity assays because the low level of expression of the cdt genes in A. actinomycetemcomitans precluded the isolation of significant quantities of biologically active gene products.
KB cells (ATCC CC17) were grown in T-75 flasks (Falcon, Becton-Dickinson Labware, Franklin Lakes, NJ) in 15 ml of growth medium containing 5% fetal bovine serum, 720 units/ml penicillin and 1 μg/ml gentamicin (αMEM-AB) in a humidified atmosphere containing 5% CO2 in air at 37°C for 6 days. The adherent cells were removed by mild treatment with trypsin, suspended in the same medium, and diluted for use in the CDT assays.
Toxicity assays were based on those used previously by Mayer et al. (17) to evaluate the activity of the CDT on Chinese hamster ovary cells. Serial dilutions of the bacterial extracts, containing 0.006–6000 ng of protein, were made in sterile tissue culture medium. Protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad, Rockville Centre, MA). Cell suspensions containing 1000 cells (3.0 ml), along with 600 μl of each dilution of filter-sterilized bacterial extracts or extract-free αMEM as a control, were added to each well of six-well tissue culture plates. Each dilution was run in triplicate. The plates were incubated for 6days at 37°C in an atmosphere containing 5% CO2. The cells were then fixed in 10% formalin and stained with crystal violet. Resulting colonies were counted both manually and automatically using an UltraLum Image Software Computer Program and Integrating Camera with a motorized zoom lens, a dual light transilluminator, and a VG-5 frame grabber (UltraLum Inc., Carson, CA). The automated counting method was used to confirm the manual counts, remove any inherent bias, and improve reproducibility in repetitive experiments. Each experiment was repeated at least once. Colony counts obtained by the two methods were nearly identical. Data were expressed as the percentage of colony forming units relative to cell cultures minus the CDT-containing extract.
Initial experiments established that KB cells were exquisitely sensitive to the CDT-containing extract from UP54, such that nanogram amounts of total soluble bacterial protein significantly reduced the survival of the cells (Fig. 2). No effect was observed with sonic extract from UP57, which contains a deletion in the CDT locus, even when 100 times more protein (6000 μg/well) was added to cell cultures. Strain UP57 is a naturally occurring CDT mutant but may not be isogenic for that locus. It is possible that other differences between UP57 and UP54 account for the differential effects on KB cells. However, this interpretation of the data is not likely based on the fact that there was an exact correlation between deletions in the cdt locus of various strains of A. actinomycetemcomitans and the absence of toxic activity in the corresponding sonicates (17). The results of this experiment also showed that extensive subculturing of UP54 in the laboratory, as required for invasion studies, did not result in the loss of CDT expression.
Fig. 2.
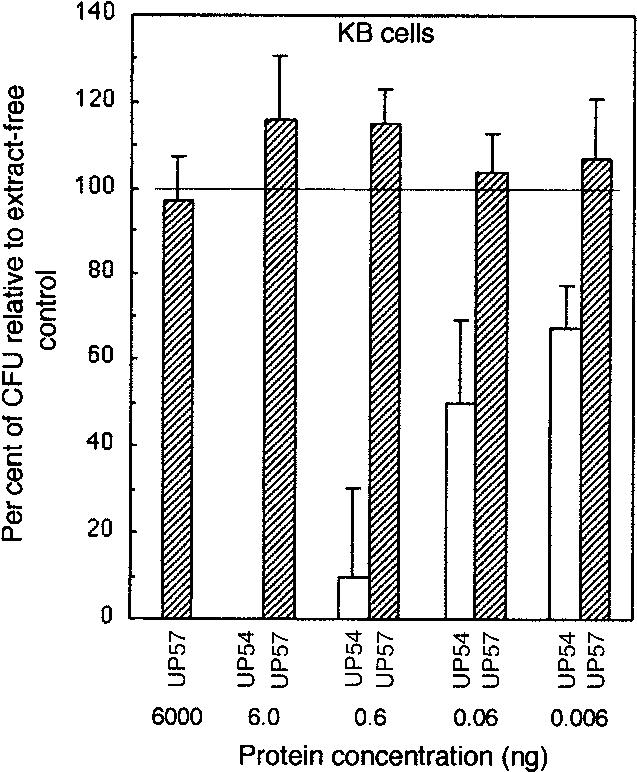
Comparison of the effects of sonic extract from A. actinomycetemcomitans UP54 (invasion positive, CDT+) and UP57 (invasion negative, CDT−) on KB cells. Cells were exposed to sonic extract ranging from 0.006 to 6.0 ng (UP54) and 0.006 ng to 6.0 μg (UP57) of total protein for 6 days. Experiments were performed in six-well plates, in triplicate, and stained colonies were counted by an automated system. Untreated KB cell cultures were used to obtain values for 100% survival (100 ± 15.2). X-axis units are in ng of protein/1000 cells.
Cellular distension is a defining property of the CDT. To determine if the morphology of the KB cells was altered following exposure to CDT-containing extract from UP54, cells were monitored as they grew in real time in the chamber of a videomicrography apparatus. The KB cells were grown in either CO2-independent medium or αMEM to observe distension. CO2-independent medium was used to grow the cells, outside the CO2 incubator, in the chamber of a Nikon incubator NP-2 videomicrography apparatus (Nikon Canada, Inc., Mississauga, Ontario). αMEM was used when cells were grown in the incubator. The cell cultures were challenged with a range of 10-fold dilutions of a sonic extract, containing 0.006–600 ng of protein, from strain UP54 at the time the cells were seeded in the tissue culture plates (time 0). Photographs of the cultures grown in CO2-independent medium were taken at 0 (Fig. 3A), 13 (Fig. 3B) and 24.5 (not shown) h of growth. In addition, cells grown in αMEM, without (Fig. 3C) and with (Fig. 3D) UP54 extract, were photographed at 25 h of growth. All photographs were taken at a magnification of 85×. As the KB cells replicated, the intercellular spaces became filled with enlarged, oddly shaped or distended cells when exposed to the UP54 extract. The change in morphology was apparent within 13 h of exposure and became more pronounced by 25 h.
Fig. 3.
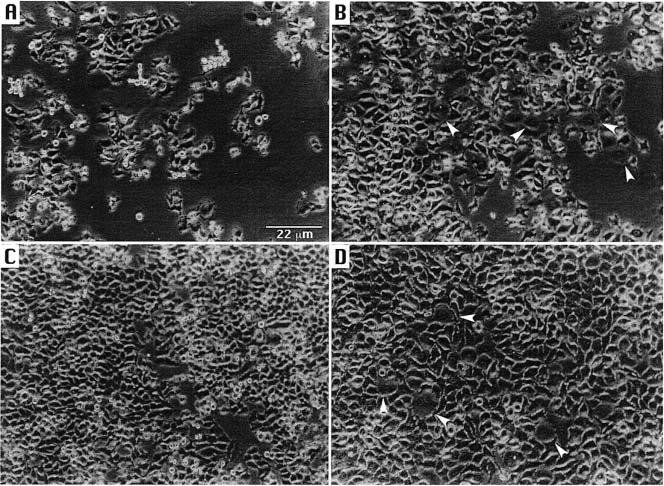
Cellular distension of KB cells following exposure to CDT-containing extract of A. actinomycetemcomitans UP54. Cells grown in CIM were photographed at times 0 (A) and 13 (B) h. Cells grown without (C) and with (D) bacterial extract in αMEM were photographed at 25 h. Bar = 22 μm. Arrows identify distended cells.
Size comparisons of the KB cells were made by digitizing the photographs and measuring the length and area of individual cells using the computer program nih image (version 1.6.1; http://rsb.info.nih.gov/nih-image/download.html). Length measurements were made at the widest cell diameter and were converted to μm using the magnification bar shown in Fig. 3A. Measurements of 10 cells, chosen at random, were averaged. KB cells grown in CO2-independent medium measured at 0 and 24.5 h, following exposure to CDT-containing extract, had average lengths of 6.1 ± 1.0 and 10.8 ± 1.8 μm, respectively, and average surface areas of 36.2 ± 12.7 and 159.5 ± 50.0 μm2, respectively. The average surface area of the KB cells treated for 24.5 h was approximately 4 times greater than that of the treated cells at exposure time 0. Similar results were obtained when the KB cells were grown under standard laboratory conditions in αMEM in a CO2 incubator. The average length of these cells was 4.0 ± 0.6 (minus CDT-containing extract) and 9.2 ± 1.8 μm (CDT-containing extract), respectively, following growth for 25 h. The untreated and treated cells had average surface areas of 30.4 ± 10.1 and 160.5 ± 65.3 μm2, respectively. The average surface area of the αMEM-grown, extract-treated KB cells was approximately 5 times greater than that of the untreated cells.
CDT-containing extract from UP54 exhibited a dose-dependent effect on the survival of KB cells as measured by a reduction in the formation of colony forming units (Fig. 4, upper panel). A TD50 was defined as the amount of bacterial extract, in mass (ng) of protein, required to reduce the survival of a cell population containing 1000 cells to 50% of that of the control. The TD50 for KB cells was in the range of 10–30 pg of total protein. This was approximately 1000-fold lower than the TD50 obtained with Chinese hamster ovary cells (17), demonstrating the increased sensitivity of the epithelial-like cells to the CDT. The TD50 values for CDT-containing extracts from strains UP6 and UP28 were approximately the same as those for the UP54 extract. These data also suggest that elevated leukotoxin expression has no effect on the cytotoxicity attributed to the CDT, as UP6 is a high-level leukotoxin-producing strain. HEp-2 cells also responded to the CDT-containing extracts in a dose-dependent fashion (Fig. 4, lower panel). However, the HEp-2 cells were less sensitive than the KB cells to the CDT-containing extracts. The TD50 for the CDT-containing extracts from all three strains was approximately 10-fold lower for KB than HEp-2 cells.
Fig. 4.
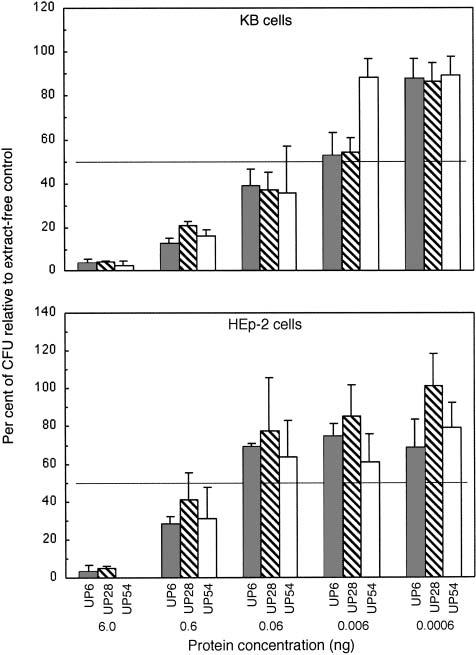
Determination of TD50 concentrations of CDT-containing extracts from A. actinomycetemcomitans. Cells were exposed to 0.0006–6.0 ng of total protein from strains UP6, UP28 and UP54 for 6 days. Upper panel, 1000 KB cells/well; lower panel, 1000 HEp-2 cells/well. Experiments were performed in six-well plates. Stained colonies were counted and data were plotted as described in the legend to Fig. 2.
The minimal exposure time required for CDT-containing extracts to elicit a cytotoxic response was determined. KB and HEp-2 cells (1000 cells/well in 3 ml of growth medium per well in six-well plates) were challenged with concentrations of sonic extract (0.06–600 ng of protein/1000 cells) from UP54 for 15, 30, 60 and 120 min after the cells were allowed to attach to the plates (overnight growth). Extract-free αMEM was added to additional wells as a control. Culture supernatants were removed by suction and 3 ml of fresh sterile medium was added to each well. All plates were incubated for a total of 6 days and the colonies fixed, stained and counted. All samples were run in triplicate. Exposure time dependence was best seen with 60 ng of total protein/1000 cells (Fig. 5). Both 60- and 120-min exposures prevented the growth/attachment of KB (Fig. 5, upper panel). Maximum growth inhibition of HEp-2 cells was not observed until 120 min of exposure to CDT-containing extract (Fig. 5, lower panel). Shorter exposure times gave a progressively increased survival rate. Exposure to 6 ng of total protein/1000 cells required longer exposure times to significantly reduce the survival of both KB and HEp-2 cells. Lower concentrations of the bacterial extract had minimal effects on cell survival. These data showed that the growth of both KB and HEp-2 cells was rapidly inhibited by the toxin and that the growth arrest was irreversible.
Fig. 5.
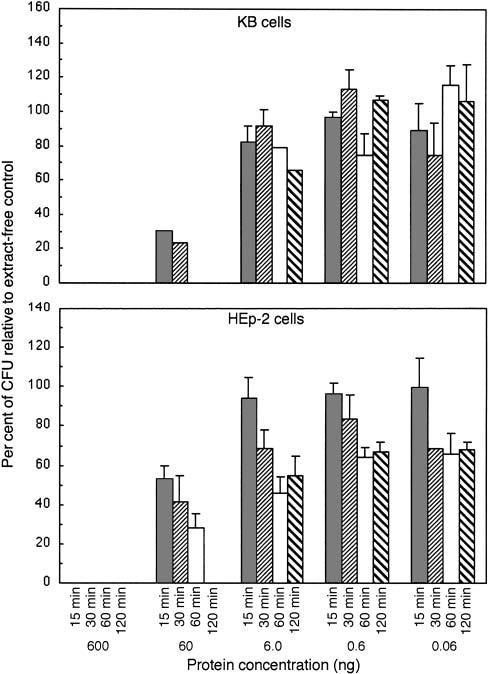
Determination of the minimal exposure time required to elicit a cytotoxic effect with sonic extracts from A. actinomycetemcomitans. CDT-containing extract (0.06, 0.6 and 6.0 of total protein) was added to cells, in triplicate wells, for 15, 30, 60 and 120 min. The cells were then placed in fresh extract-free medium and incubated for 6 days. Upper panel, 1000 KB cells/well; lower panel, 1000 HEp-2 cells/well. Cells were fixed and stained and the colonies counted by computer as described in the legend to Fig. 2.
The kinetics of the effect of CDT-containing sonic extract on the survival of attached and growing HEp-2 cells was also examined (Fig. 6). Cells were challenged with 0.06, 0.6 or 6.0 ng of protein/1000 cells of UP54 extract (600 μl/well) after 1–5 days of growth. All plates were incubated for a total of 8 days, after which the cells were fixed, stained and counted. The clearest trends were obtained with 6 ng of total protein/1000 cells of bacterial extract. There was an inverse effect of the toxin on cell survival. The HEp-2 cells became more resistant to the effects of the toxin as the age of the cell culture increased. The same amount of CDT-containing extract had no effect on cell survival after 5 days of growth. Cultures were routinely grown for 8 days.
Fig. 6.
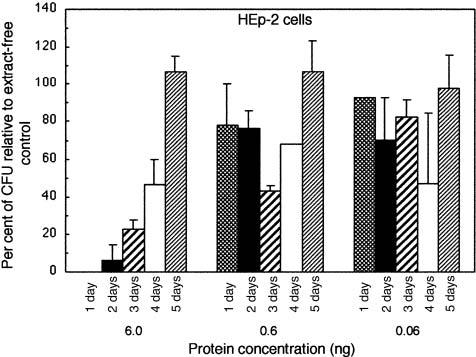
Effect of sonic extract from strain UP54 on the survival of HEp-2 cells. CDT-containing extract (0.06, 0.6 and 6.0 of total protein) was added to cells, in triplicate wells, on days 1, 2, 3, 4 and 5 of growth. All plates were incubated for a total of 8 days, after which the cells were fixed and stained and the colonies counted by computer. Data were plotted as described in the legend to Fig. 2.
To date, basic studies of the effects of the native (non-recombinant) A. actinomycetemcomitans CDT have been performed with Chinese hamster ovary (17), HeLa (28, 29), and human T cells (28). However, detailed kinetic studies of toxicity and real time effects on cell morphology have not yet been reported with these cells. Other studies have used recombinant proteins to examine the biological activity of the A. actinomycetemcomitans CDT (1). In the present study, we examined the kinetics of the effects of the native CDT, from an invasive strain of A. actinomycetemcomitans, on KB and HEp-2 cells. We found that epithelial cells are very sensitive to the CDT and that the cytotoxic effects are relatively rapid and irreversible. The KB cell line originally derived from an epidermoid carcinoma of the mouth is especially relevant because of its extensive use in A. actinomycetemcomitans invasion studies (3, 16, 18). The HEp-2 cell line was reportedly derived from a laryngeal epidermoid carcinoma and has also been used for invasion studies (3). Both of these cells lines were exquisitely sensitive to the CDT-containing extracts from A. actinomycetemscomitans. The HEp-2 cells were less sensitive, by an order of magnitude, to the effects of the CDT-containing extracts.
Distension of cells following brief exposure to sonic extract from strain UP54 may be a consequence of the effects of stretching of the cells while attached to the substratum (tissue culture plate). Flow cytometry data indicated no difference in cell size from baseline when the KB cells were suspended in medium following exposure to a CDT-containing extract. Furthermore, both CDT-treated and untreated KB cells showed no difference in cell volume. It has been reported that the CDT fraction from A. actinomycetemcomitans is immunosuppressive and causes the distension of HeLa cells but not T cells (28). It is possible that the distension of epithelial cells may be a function of the way cell cycle blockade affects the turnover of cytoskeletal elements that determine cell shape and the interface with the extracellular matrix and substratum in vitro. Therefore, epithelial cells may be more sensitive than T cells to the morphological outcomes of CDT toxicity. In this regard, CDT-treated Chinese hamster ovary cells grown on tissue culture plates showed an accumulation of F-actin filaments that were thought to resemble stress fibers (2).
The cytotoxic effects of CDT-containing bacterial extracts (600 ng of total protein) were provoked by as little as 15 min of contact with both KB and HEp-2 cells. Cell cultures could not recover from the effects of the CDT even though the plates were thoroughly washed following the 15-min exposure. If HEp-2 cells were challenged with CDT-containing material after 5 days of growth, there was no apparent cytotoxic effect. These results indicated that, at least in vitro, confluent cell layers may limit the accessibility of the cells to the toxin. It is possible that there was toxin limitation as a result of the relatively large number of cells in the culture following 5 days of growth.
The effects of the CDT on cultured KB and Hep-2 cells may not be apparent during cell invasion, performed in vitro, because of the short exposure times used in such assays. However, studies designed to determine the persistence and replication of A. actinomycetemcomitans in epithelial cells should take into consideration potential cytotoxic effects. CDT activity may even favor conditions for bacterial growth within epithelial cells once the bacteria have invaded. These effects may be even more pronounced in vivo, where time of interaction or exposure of epithelial cells to A. actinomycetemcomitans or its secreted products may be significantly prolonged. As in the case of the leukotoxin, the expression of this little-known factor may have several cell modulating functions. In addition, expression of a functional CDT extends the repertoire of virulence potentials of A. actinomycetemcomitans by increasing the ways in which this bacterium can negatively interact with orally relevant human cells.
Acknowledgments
This study was supported by USPHS grant RO1-DE12593 to JMD and Hospital for Sick Children Foundation grant XG 95-036 and MRC Canada grant MT-5619 to RPE.
References
- 1.Akifusa S, Poole S, Lewthwaite J, Henderson B, Nair SP. Recombinant Actino-bacillus actinomycetemcomitans cyto-lethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect Immun. 2001;69:5925–5930. doi: 10.1128/IAI.69.9.5925-5930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon V, Khao C, Dreyfus LA. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blix IJS, Hars R, Preus HR, Helgeland K. Entrance of Actinobacillus actinomycetemcomitans into HEp-2 cells in vitro. J Periodontol. 1992;63:723–728. doi: 10.1902/jop.1992.63.9.723. [DOI] [PubMed] [Google Scholar]
- 4.Bueno LC, Mayer MPA, DiRienzo JM. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J Periodontol. 1998;69:998–1007. doi: 10.1902/jop.1998.69.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn J, Leong JM. Arresting features of bacterial toxins. Science. 2000;290:287–288. doi: 10.1126/science.290.5490.287. [DOI] [PubMed] [Google Scholar]
- 6.Comayras C, Tasca C, Pérès SY, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope LD, Lumbley S, Latimer JL, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Invest. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes-Bratti X, Karlsson C, Lagergard T, Thelestam M, Frisan T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J Biol Chem. 2001;276:5269–5302. doi: 10.1074/jbc.M008527200. [DOI] [PubMed] [Google Scholar]
- 10.DiRienzo JM, McKay TL. Identification and characterization of genetic cluster groups of A. actinomycetemcomitans isolated from the human oral cavity. J Clin Microbiol. 1994;32:75–81. doi: 10.1128/jcm.32.1.75-81.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiRienzo JM, Slots J, Sixou M, Sol MA, Harmon R, McKay TL. Specific genetic variants of A. actinomycetemcomitans correlate with disease and health in a regional population of localized juvenile periodontitis families. Infect Immun. 1994;62:3058–3065. doi: 10.1128/iai.62.8.3058-3065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elwell CA, Dreyfus LA. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson WM, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microbial Path. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 14.Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Tejero M, Galán JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 16.Lépine G, Caudry S, DiRienzo JM, Ellen RP. Epithelial cell invasion by Actinobacillus actinomycetemcomitans strains from restriction fragment-length polymorphism groups associated with juvenile periodontitis or carrier status. Oral Microbiol Immunol. 1998;13:341–347. doi: 10.1111/j.1399-302x.1998.tb00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer MPA, Bueno LC, Hansen EJ, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer DH, Lippmann JE, Fives-Taylor P. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer DH, Sreenivasan PK, Fives-Taylor P. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oguchi M, Ishisaki A, Okahashi N, et al. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in the G2/M phase and apoptosis. Infect Immun. 1998;66:5980–5987. doi: 10.1128/iai.66.12.5980-5987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathol. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 22.Pérès SY, Marchès O, Daigle F, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 23.Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickett CL, Whitehouse CA. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 26.Scott DA, Kaper JB. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenker BJ, Hoffmaster RH, McKay TL, Demuth DR. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 28.Shenker BJ, McKay TL, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 29.Sugai M, Kawamoto T, Pérès SY, et al. Cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL. Camphylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]