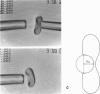
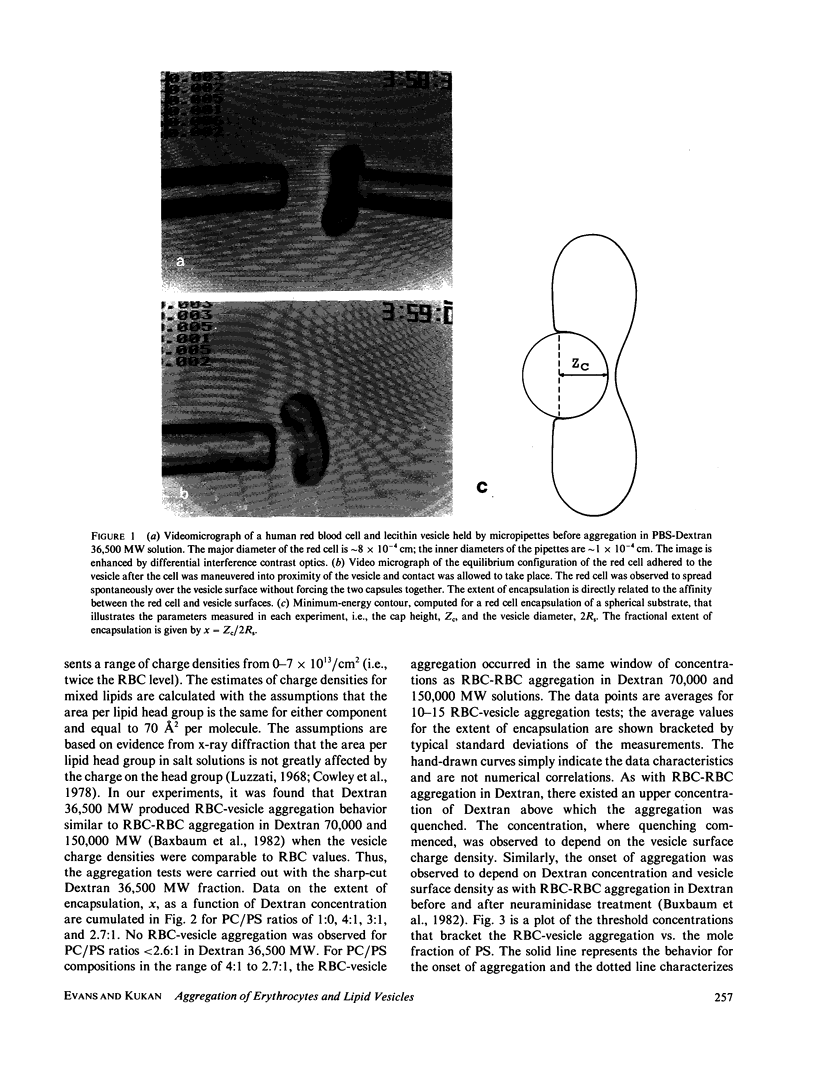
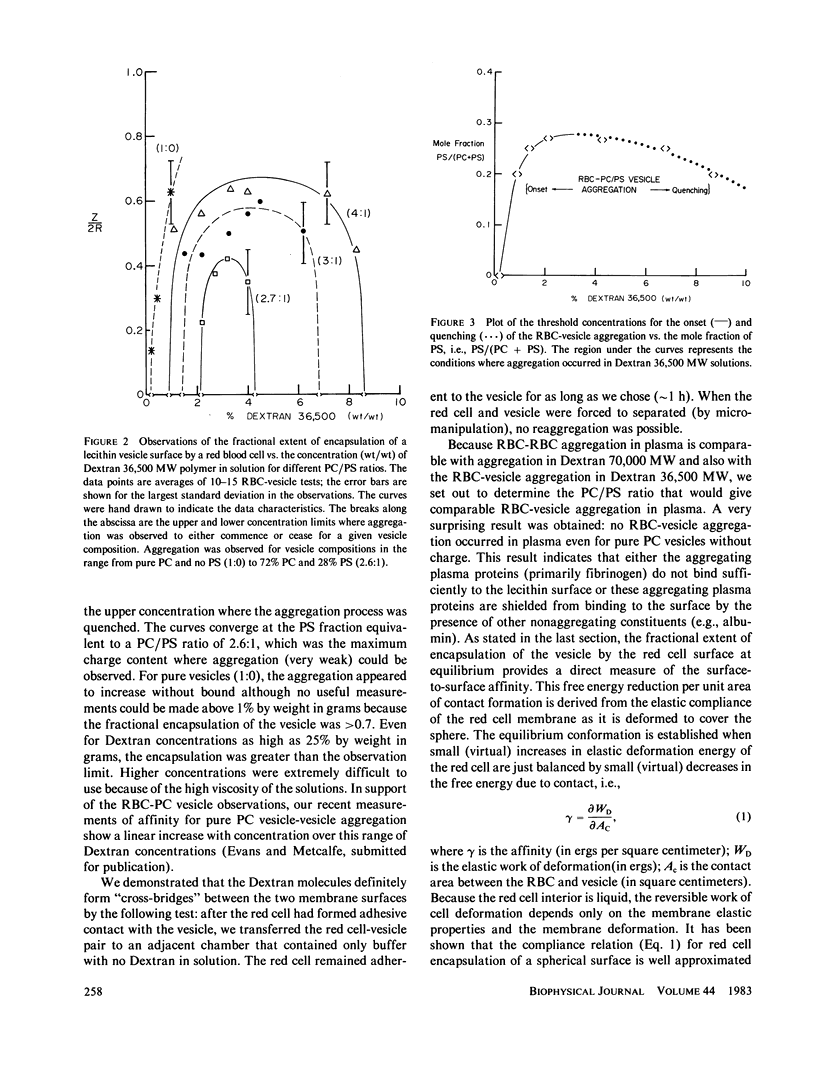
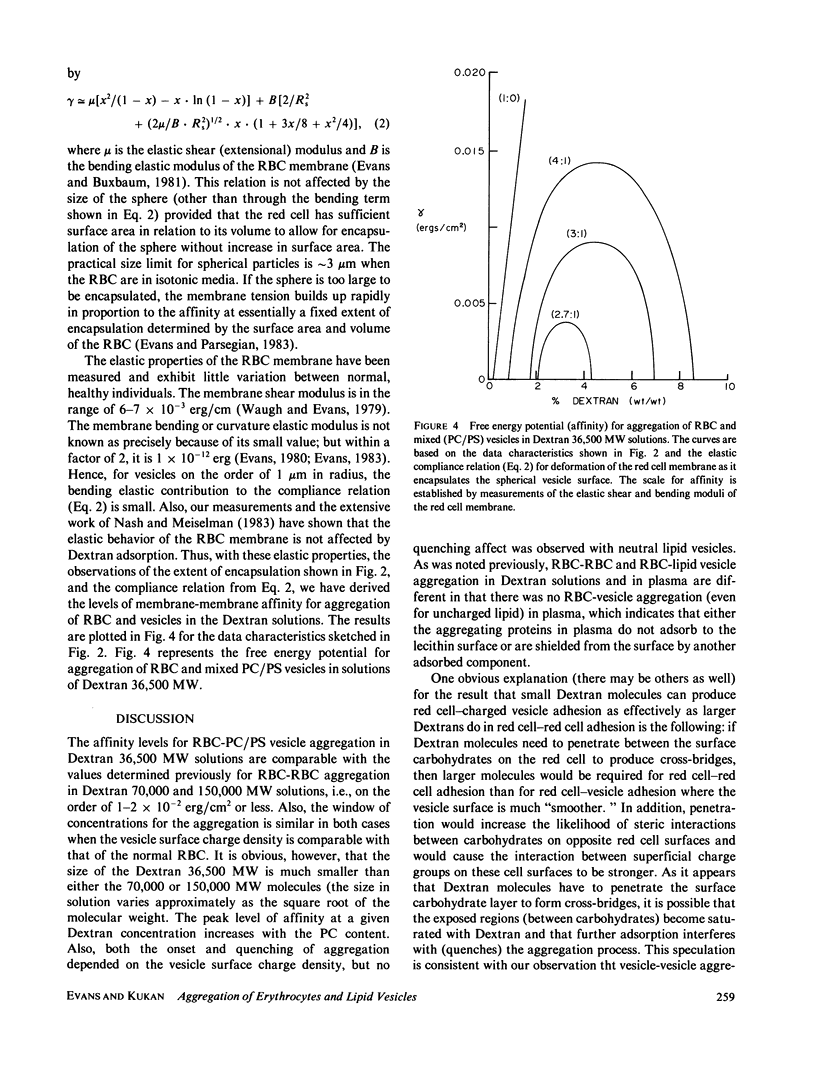
Abstract
The free energy potential (affinity) for aggregation of human red blood cells and lipid vesicles in Dextran solutions and blood plasma has been quantitated by measuring to what extent a vesicle is encapsulated by the red cell surface. The free energy reduction per unit area of contact formation (affinity) was computed from the observation of the fractional extent of encapsulation at equilibrium with the use of a relation based on the elastic compliance of the red cell membrane as it is deformed to adhere to the vesicle surface. Micromanipulation methods were used to select and transfer single lipid vesicles (2-3 X 10(-4) cm diameter) from a chamber that contained the vesicle suspension to a separate chamber on the microscope stage that contained red cells in an EDTA buffer with Dextran or whole plasma. The vesicle and a red cell were maneuvered into close proximity and contact allowed to take place without forcing the cells together. To evaluate the effects of surface charge density and steric interactions on aggregation, vesicles were made from mixtures of egg phosphatidylcholine (PC) and bovine phosphatidylserine (PS) over a range of mole ratios (PC/PS)from (1:0) to (1:1); the vesicles were formed by rehydration in buffer. The Dextran solutions were made with a sharp-cut fraction of 36,500 MW in a concentration range of 0-10% by weight in grams (wt/wt). It was found that the Dextran 36,500 MW fraction produced aggregation behavior for red cells and vesicles similar to red cell-red cell aggregation in Dextran 70,000-150,000 MW fractions, when the vesicle surface charge density was comparable with that of normal red cells (i.e., PC/PS ratio of -3:1). This result indicated that Dextran molecules penetrate between the carbohydrate groups on the red cell surface and that either steric interactions between cell surface carbohydrates are important or many of the charge groups on red cells are superficial. Electrostatic repulsion effects were apparent, as no aggregation in Dextran 36,500 MW occurred for PC/PS ratios <2.6:1; the level of affinity increased with the PC content at a specific Dextran concentration. Affinities were measured in the range of 0-2 x 10-2 ergs/cm2. When adherent red cell-vesicle pairs were transferred into a Dextran-free buffer, the pair did not spontaneously separate.They maintained adhesive contact until forcibly parted, after which they would not read here. This demonstrates that Dextran forms a "cross-bridge" between the membrane surfaces. Red cell-vesicle aggregation was also tested in whole plasma, which normally yields affinity values in the range of 2-4 x 10-3 ergs/cm2 for red cell-red cell aggregation.However, no red cell-vesicle aggregation occurred in plasma, even for pure PC vesicles. This result indicates that either the aggregating plasma proteins (primarily fibrinogen) do not bind sufficiently to the lecithin surface, or they are shielded from binding to the surface by the presence of other nonaggregating components (perhaps albumin).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxbaum K., Evans E., Brooks D. E. Quantitation of surface affinities of red blood cells in dextran solutions and plasma. Biochemistry. 1982 Jun 22;21(13):3235–3239. doi: 10.1021/bi00256a032. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983 Jul;43(1):27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Minimum energy analysis of membrane deformation applied to pipet aspiration and surface adhesion of red blood cells. Biophys J. 1980 May;30(2):265–284. doi: 10.1016/S0006-3495(80)85093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Buxbaum K. Affinity of red blood cell membrane for particle surfaces measured by the extent of particle encapsulation. Biophys J. 1981 Apr;34(1):1–12. doi: 10.1016/S0006-3495(81)84834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Seaman G. V. Electrokinetic studies on the ultrastructure of the human erythrocyte. I. Electrophoresis at high ionic strengths--the cell as a polyanion. Arch Biochem Biophys. 1967 Oct;122(1):126–136. doi: 10.1016/0003-9861(67)90131-2. [DOI] [PubMed] [Google Scholar]
- Jan K. M., Chien S. Role of surface electric charge in red blood cell interactions. J Gen Physiol. 1973 May;61(5):638–654. doi: 10.1085/jgp.61.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Levine M., Sharp K. A., Brooks D. E. Theory of the electrokinetic behavior of human erythrocytes. Biophys J. 1983 May;42(2):127–135. doi: 10.1016/S0006-3495(83)84378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman G. V., Knox R. J., Nordt F. J., Regan D. H. Red cell aging. I. Surface charge density and sialic acid content of density-fractionated human erythrocytes. Blood. 1977 Dec;50(6):1001–1011. [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]