Abstract
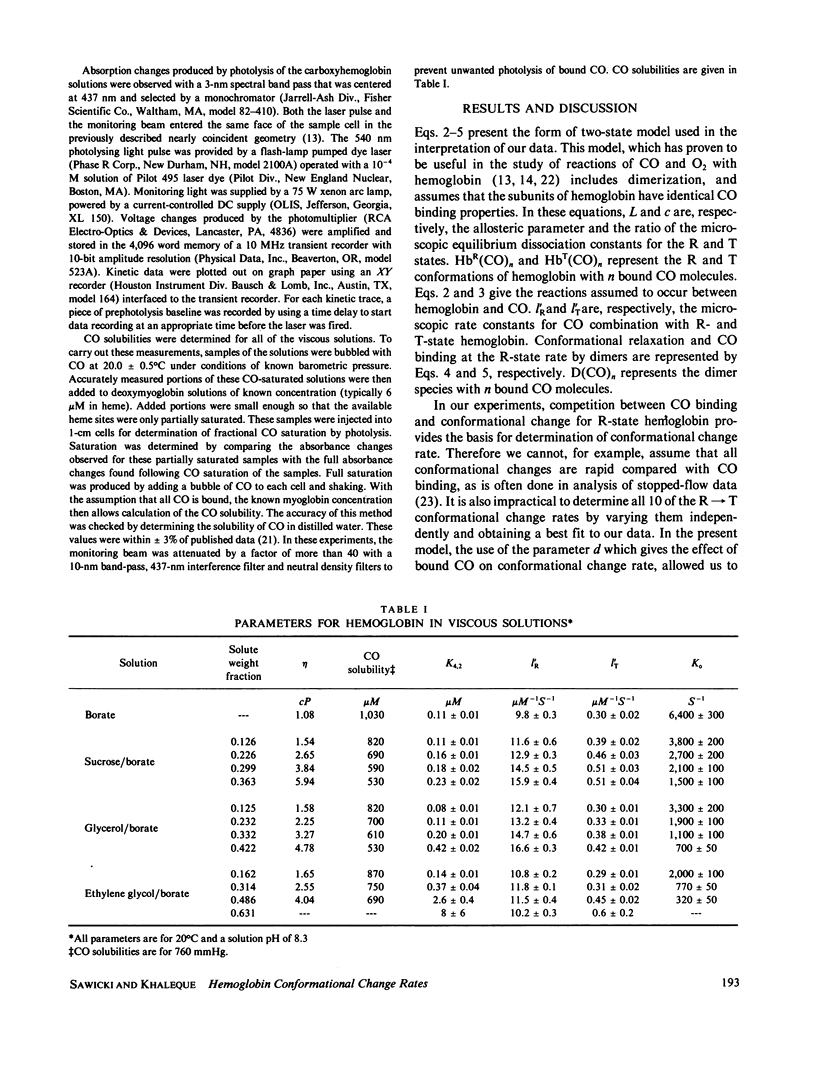
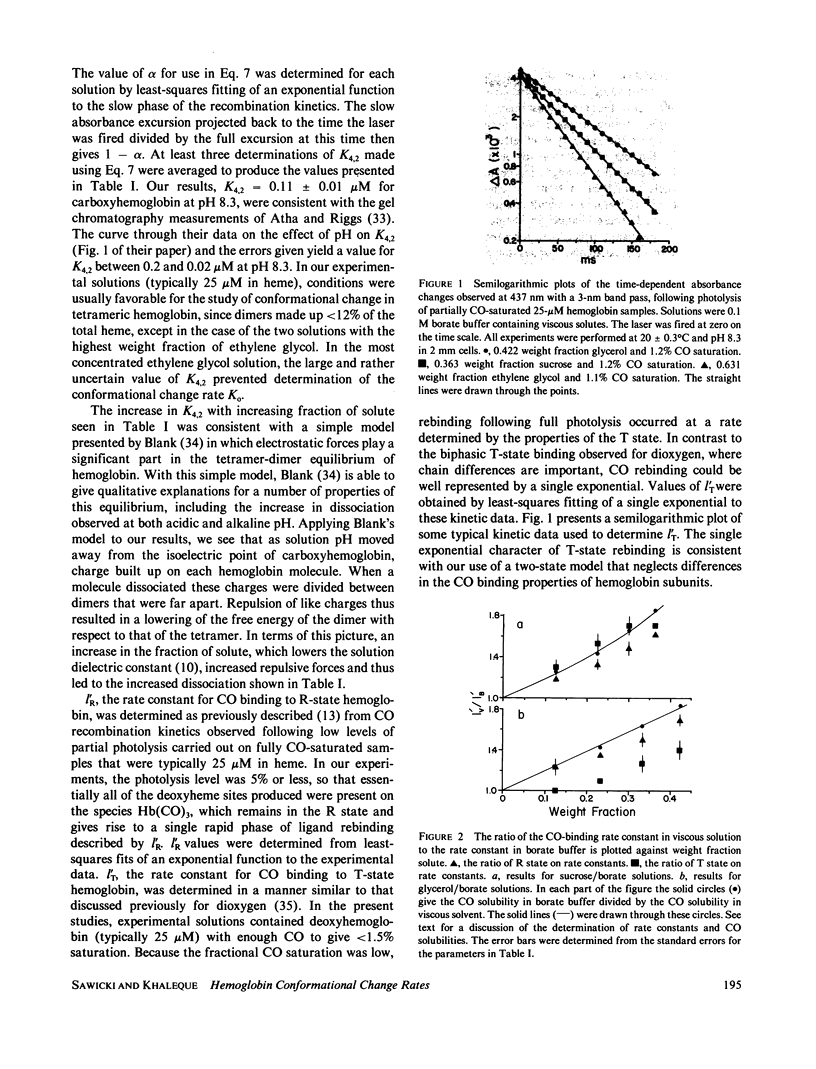
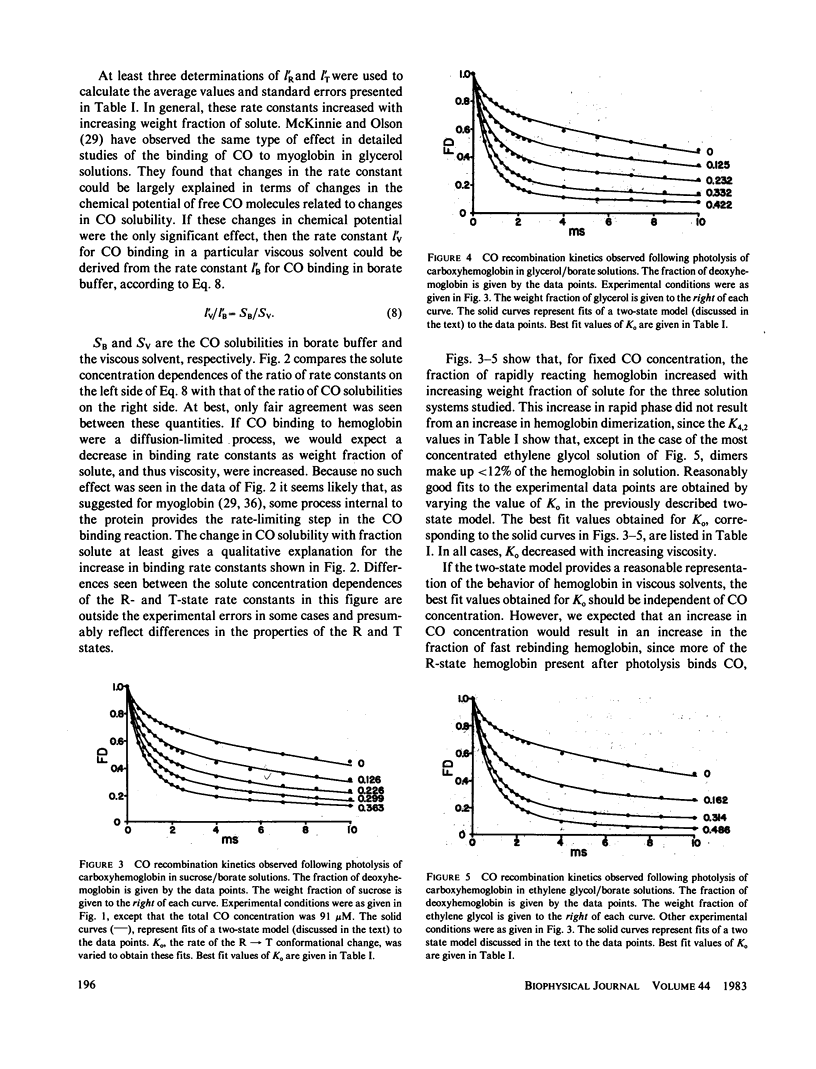
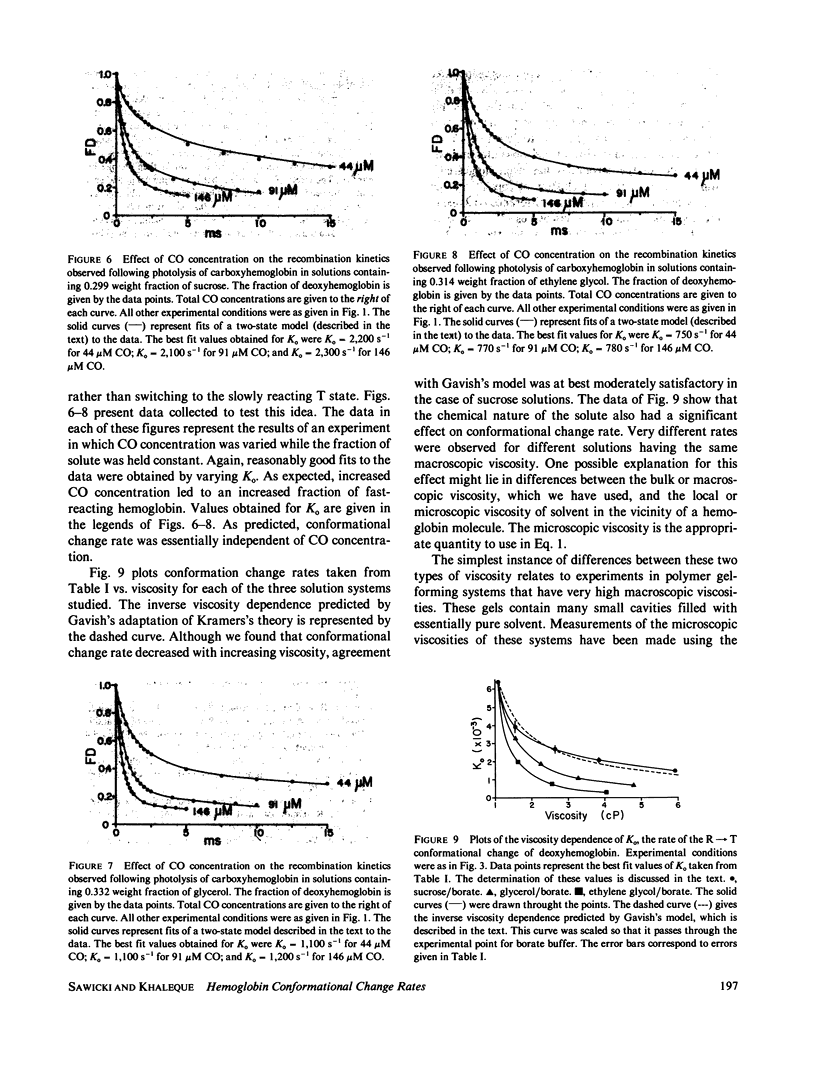
Rates for the R leads to T conformational change of deoxyhemoglobin formed by laser photolysis of carboxyhemoglobin were determined from CO rebinding observed in three solution systems with viscosities between 1 and 6 cP. Experiments were carried out at 20 degrees C and pH 8.3 in solutions consisting of borate buffer containing various amounts of sucrose, glycerol, or ethylene glycol. As in the case of earlier experiments in borate buffer (Sawicki and Gibson, 1976, J. Biol. Chem., 251:1533-1542), a simple two-state allosteric model which takes into account tetramer-dimer dissociation was found to give a good description of all experimental results. Using measured values for the R- and T-state CO-binding rate constants and the tetramer-dimer dissociation constant, values for the conformational change rate were determined by fitting this model to the experimental data. These rates were compared with Gavish's transient strain model (Gavish, 1978, Biophys. Struct. Mech., 4:37-52), which predicts an inverse dependence of conformational change rate on viscosity. Although fair agreement is found for hemoglobin in sucrose/borate solutions, in glycerol/borate and ethylene glycol/borate solutions, conformational change rate falls off much more rapidly with increasing viscosity than predicted by the model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. E., Moffat J. K., Gibson Q. H. The kinetics of ligand binding and of the association-dissociation reactions of human hemoglobin. Properties of deoxyhemoglobin dimers. J Biol Chem. 1971 May 10;246(9):2796–2807. [PubMed] [Google Scholar]
- Arakawa T., Timasheff S. N. Stabilization of protein structure by sugars. Biochemistry. 1982 Dec 7;21(25):6536–6544. doi: 10.1021/bi00268a033. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Riggs A. Tetramer-dimer dissociation in homoglobin and the Bohr effect. J Biol Chem. 1976 Sep 25;251(18):5537–5543. [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Coppey M., Tourbez H., Valat P., Alpert B. Study of haem structure of photo-deligated haemoglobin by picosecond resonance Raman spectra. Nature. 1980 Apr 10;284(5756):568–570. doi: 10.1038/284568a0. [DOI] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish B. The role of geometry and elastic strains in dynamic states of proteins. Biophys Struct Mech. 1977 Dec 27;4(1):37–52. doi: 10.1007/BF00538839. [DOI] [PubMed] [Google Scholar]
- Gavish B., Werber M. M. Viscosity-dependent structural fluctuations in enzyme catalysis. Biochemistry. 1979 Apr 3;18(7):1269–1275. doi: 10.1021/bi00574a023. [DOI] [PubMed] [Google Scholar]
- Gray R. D. Quaternary structure of partially liganded intermediates of sheep carbon monoxide hemoglobin at alkaline pH. J Biol Chem. 1975 Jan 25;250(2):790–792. [PubMed] [Google Scholar]
- Gray R. D. The effect of 2,3-diphosphoglycerate on the tetramer-dimer equilibrium of liganded hemoglobin. J Biol Chem. 1974 May 10;249(9):2879–2885. [PubMed] [Google Scholar]
- Hare F., Lussan C. Variations in microviscosity values induced by different rotational behaviour of fluorescent probes in some aliphatic environments. Biochim Biophys Acta. 1977 Jun 2;467(2):262–272. doi: 10.1016/0005-2736(77)90201-2. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J., Shulman R. G., Ogawa S. An allosteric model of hemoglobin. I. Kinetics. J Mol Biol. 1971 Oct 28;61(2):425–443. doi: 10.1016/0022-2836(71)90391-3. [DOI] [PubMed] [Google Scholar]
- Kellett G. L., Gutfreund H. Reactions of haemoglobin dimers after ligand dissociation. Nature. 1970 Aug 29;227(5261):921–926. doi: 10.1038/227921a0. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- McKinnie R. E., Olson J. S. Effects of solvent composition and viscosity on the rates of CO binding to heme proteins. J Biol Chem. 1981 Sep 10;256(17):8928–8932. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Properties of the T state of human oxyhemoglobin studies by laser photolysis. J Biol Chem. 1977 Nov 10;252(21):7538–7547. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J Biol Chem. 1976 Mar 25;251(6):1533–1542. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human oxyhemoglobin studied by laser photolysis. J Biol Chem. 1977 Aug 25;252(16):5783–5788. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Tetramer-dimer dissociation of carboxyhemoglobin in the absence of dithionite. Biophys J. 1981 Aug;35(2):265–270. doi: 10.1016/S0006-3495(81)84788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. The relation between carbon monoxide binding and the conformational change of hemoglobin. Biophys J. 1978 Oct;24(1):21–33. doi: 10.1016/S0006-3495(78)85328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V. S., Schmidt M. R., Ranney H. M. Dissociation of CO from carboxyhemoglobin. J Biol Chem. 1976 Jul 25;251(14):4267–4272. [PubMed] [Google Scholar]
- Shulman R. G., Hopfield J. J., Ogawa S. Allosteric interpretation of haemoglobin properties. Q Rev Biophys. 1975 Jul;8(3):325–420. doi: 10.1017/s0033583500001840. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Edelstein S. J. Observation of the dissociation of unliganded hemoglobin. J Biol Chem. 1972 Dec 25;247(24):7870–7874. [PubMed] [Google Scholar]
- Wiedermann B. L., Olson J. S. Acceleration of tetramer formation by the binding of inositol hexaphosphate to hemoglobin dimers. J Biol Chem. 1975 Jul 10;250(13):5273–5275. [PubMed] [Google Scholar]


