Abstract
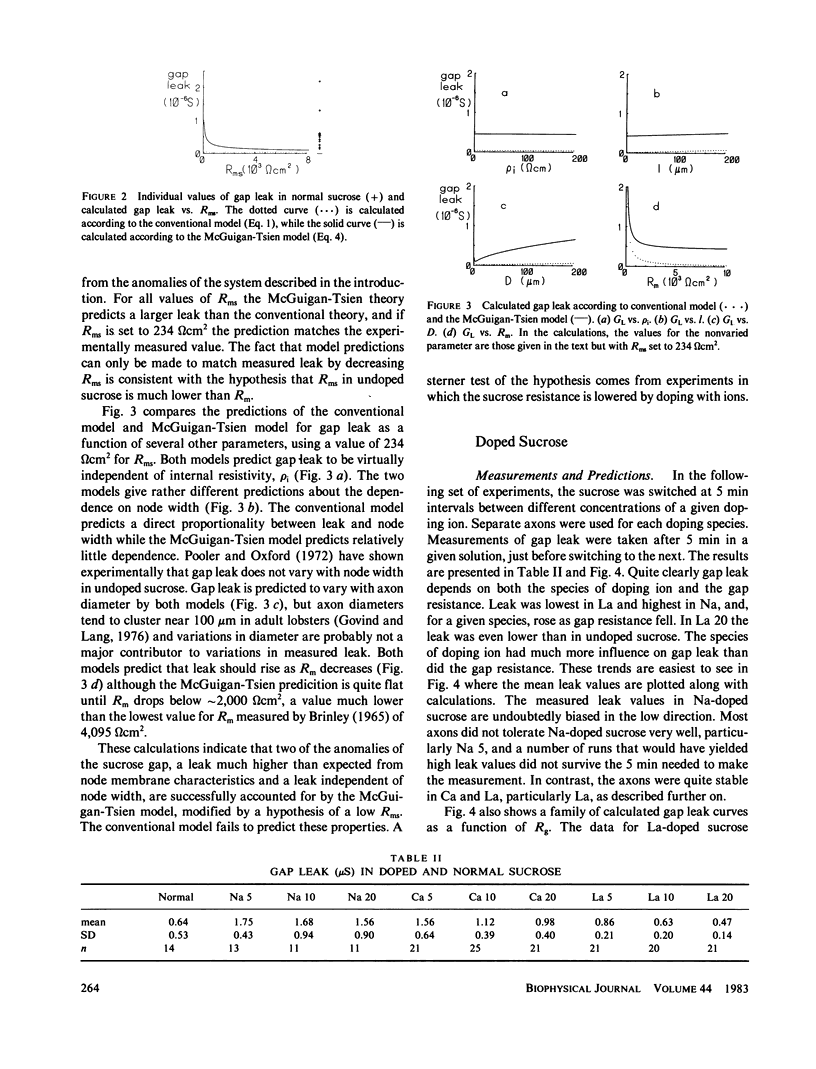
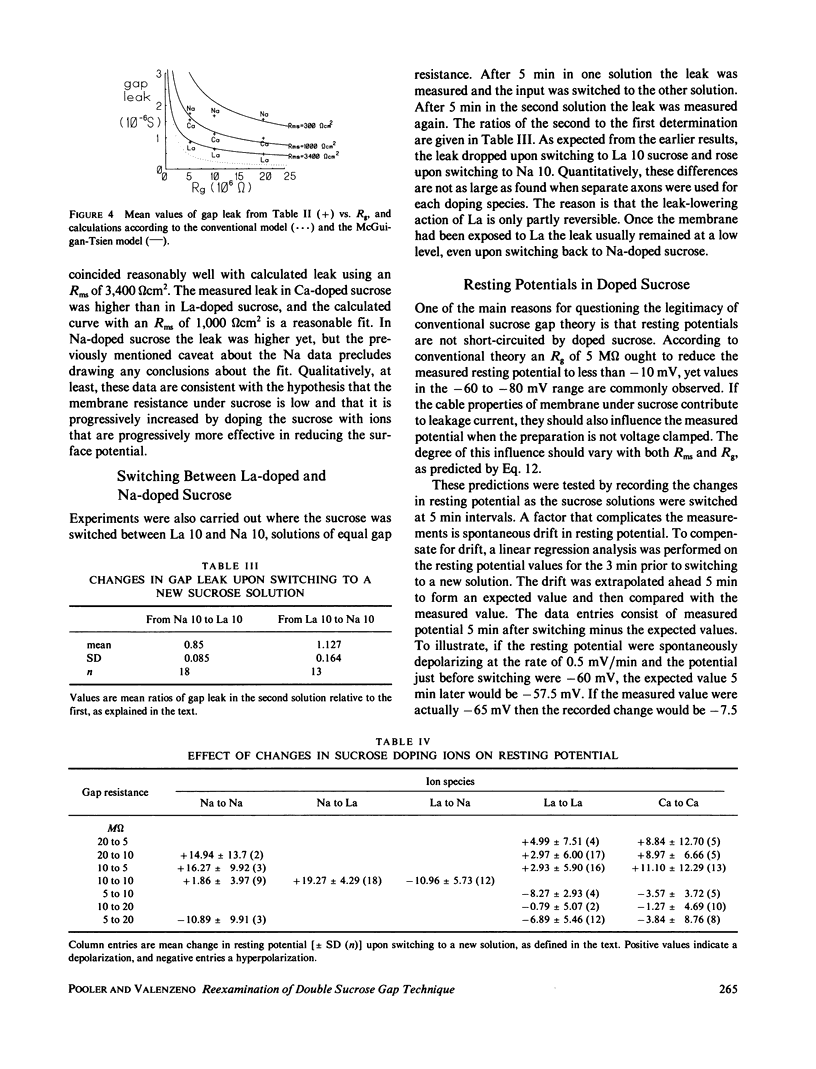
The double sucrose gap technique for the study of lobster giant axons has been reexamined. The leakage behavior of the system cannot be successfully modeled by conventional sucrose gap theory, but is accounted for by the McGuigan-Tsien model that takes into account the cable properties of membrane under sucrose. The facts of high-leakage conductance and the ability to maintain large resting potentials in the face of low sucrose gap resistance lead to a hypothesis that membrane resistance under sucrose is very low because of a large negative surface potential. Computer simulations of the leakage behavior of the conventional gap model and the McGuigan-Tsien model were compared with experimental measurements on lobster axons using normal sucrose or sucrose doped with Na+, Ca2+ or La3+ ions. As the concentration of doping ion increased, the leakage rose, but the species of doping ion had more influence on leakage than gap resistance. At equal gap resistance, leakage decreased with an increase in valence of the doping species. Leakage was even lower in La-doped sucrose at 20 M omega gap resistance than in normal sucrose at 200 M omega gap resistance. Resting potentials decreased with decreasing gap resistance and increasing valence of the doping species. Resting potential behavior was successfully simulated with a hybrid model consisting of a point node flanked by infinite cables and a shunt between ground and the voltage-measuring pool. The data support the hypothesis that the membrane resistance under sucrose is low and that it can be raised by doping the sucrose with multivalent cations, with La3+ being particularly effective. Both the leak conductance and resting potential are influenced more by membrane under sucrose than membrane in the node. The experiments also demonstrate that doping with La3+ vastly improves the stability and longevity properties of the lobster axon preparation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., Jr The effect of external calcium and magnesium depletion on single nerve fibers. J Gen Physiol. 1956 May 20;39(5):753–772. doi: 10.1085/jgp.39.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINLEY F. J., Jr SODIUM, POTASSIUM, AND CHLORIDE CONCENTRATIONS AND FLUXES IN THE ISOLATED GIANT AXON OF HOMARUS. J Neurophysiol. 1965 Jul;28:742–772. doi: 10.1152/jn.1965.28.4.742. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., McGuigan J. A. Voltage clamping of multicellular myocardial preparations: capabilities and limitations of existing methods. Prog Biophys Mol Biol. 1978;34(3):219–254. doi: 10.1016/0079-6107(79)90019-1. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Origin of axon membrane hyperpolarization under sucrose-gap. Biophys J. 2008 Dec 31;6(4):453–470. doi: 10.1016/S0006-3495(66)86669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R. A., Zak R. Calcium-induced cell death: susceptibility of cardiac myocytes is age-dependent. Science. 1981 Sep 25;213(4515):1508–1511. doi: 10.1126/science.7280671. [DOI] [PubMed] [Google Scholar]
- Crevey B. J., Langer G. A., Frank J. S. Role of Ca2+ in maintenance of rabbit myocardial cell membrane structural and functional integrity. J Mol Cell Cardiol. 1978 Dec;10(12):1081–1100. doi: 10.1016/0022-2828(78)90354-1. [DOI] [PubMed] [Google Scholar]
- DALTON J. C. Effects of external ions on membrane potentials of a lobster giant axon. J Gen Physiol. 1958 Jan 20;41(3):529–542. doi: 10.1085/jgp.41.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind C. K., Lang F. Growth of lobster giant axons: correlation between conduction velocity and axon diameter. J Comp Neurol. 1976 Dec 15;170(4):421–433. doi: 10.1002/cne.901700403. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Membrane potentials of the lobster giant axon obtained by use of the sucrose-gap technique. J Gen Physiol. 1962 Jul;45:1195–1216. doi: 10.1085/jgp.45.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirounek P., Straub R. W. The potential distribution and the short-circuiting factor in the sucrose gap. Biophys J. 1971 Jan;11(1):1–10. doi: 10.1016/S0006-3495(71)86191-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE J. W., NARAHASHI T., ULBRICHT W. SODIUM CONDUCTANCE SHIFT IN AN AXON INTERNALLY PERFUSED WITH A SUCROSE AND LOW-POTASSIUM SOLUTION. J Physiol. 1964 Aug;172:163–173. doi: 10.1113/jphysiol.1964.sp007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE J. W., ULBRICHT W., TAKATA M. EFFECT OF ETHANOL ON THE SODIUM AND POTASSIUM CONDUCTANCES OF THE SQUID AXON MEMBRANE. J Gen Physiol. 1964 Nov;48:279–295. doi: 10.1085/jgp.48.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Pooler J. P. Selective modification of sodium channel gating in lobster axons by 2, 4, 6-trinitrophenol: Evidence for two inactivation mechanisms. J Gen Physiol. 1975 Dec;66(6):765–779. doi: 10.1085/jgp.66.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler J. P., Oxford G. S. Low membrane resistance in sucrose gap--a parallel leakage path. Biochim Biophys Acta. 1972 Feb 11;255(2):681–684. doi: 10.1016/0005-2736(72)90171-x. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]



