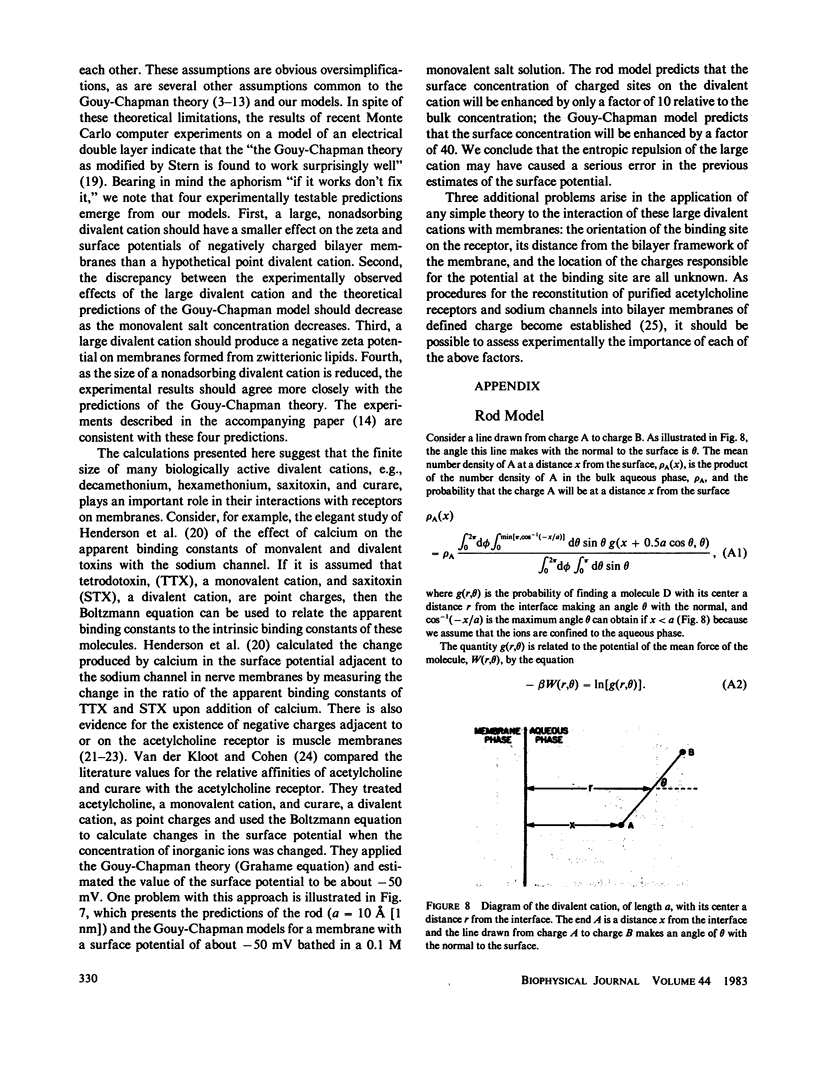
Abstract
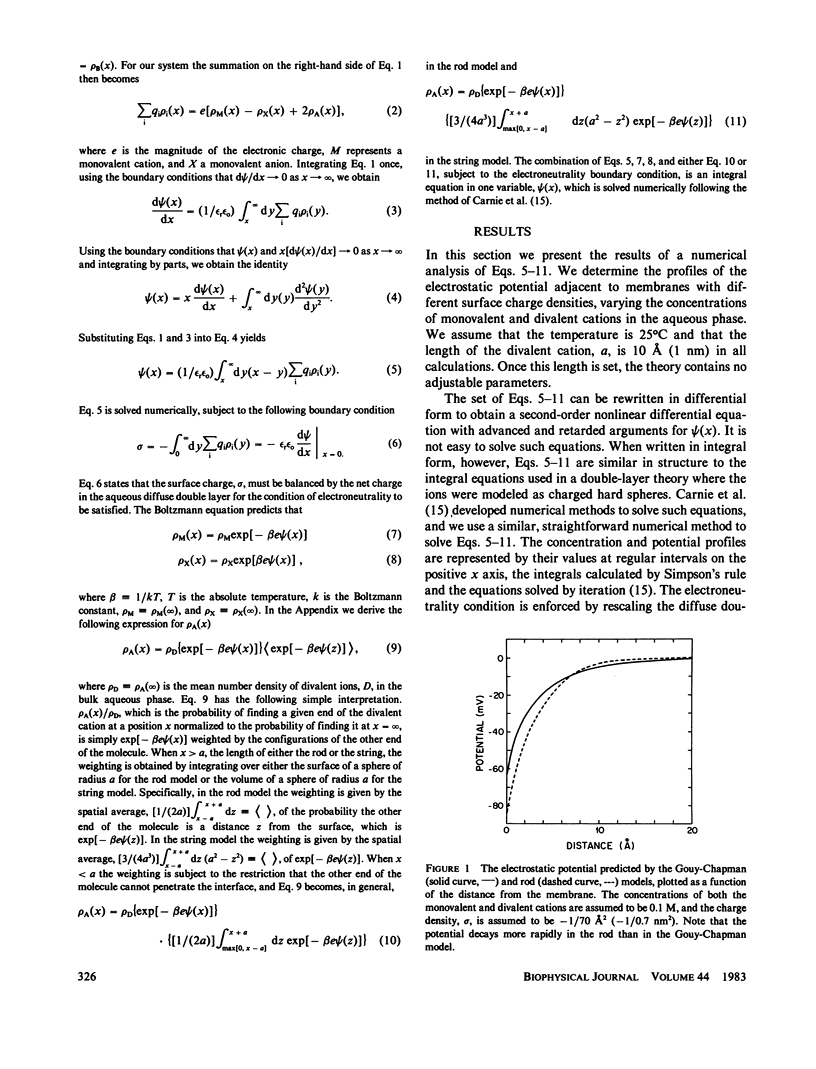
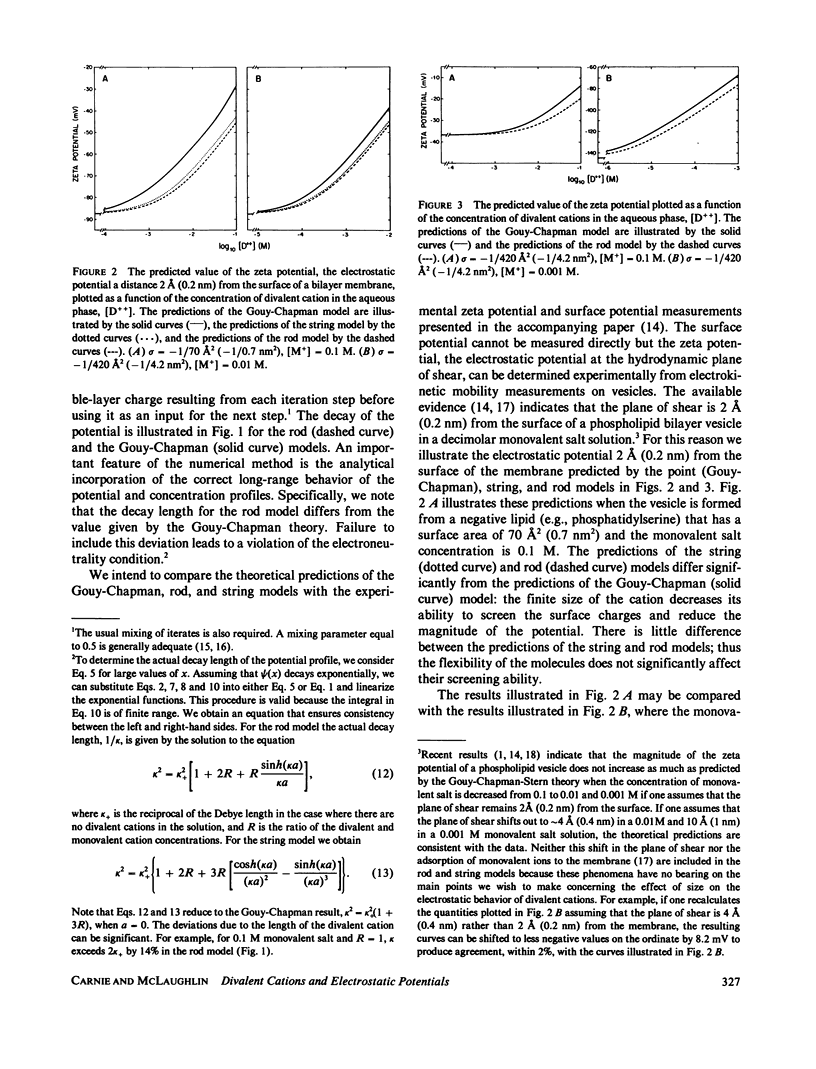
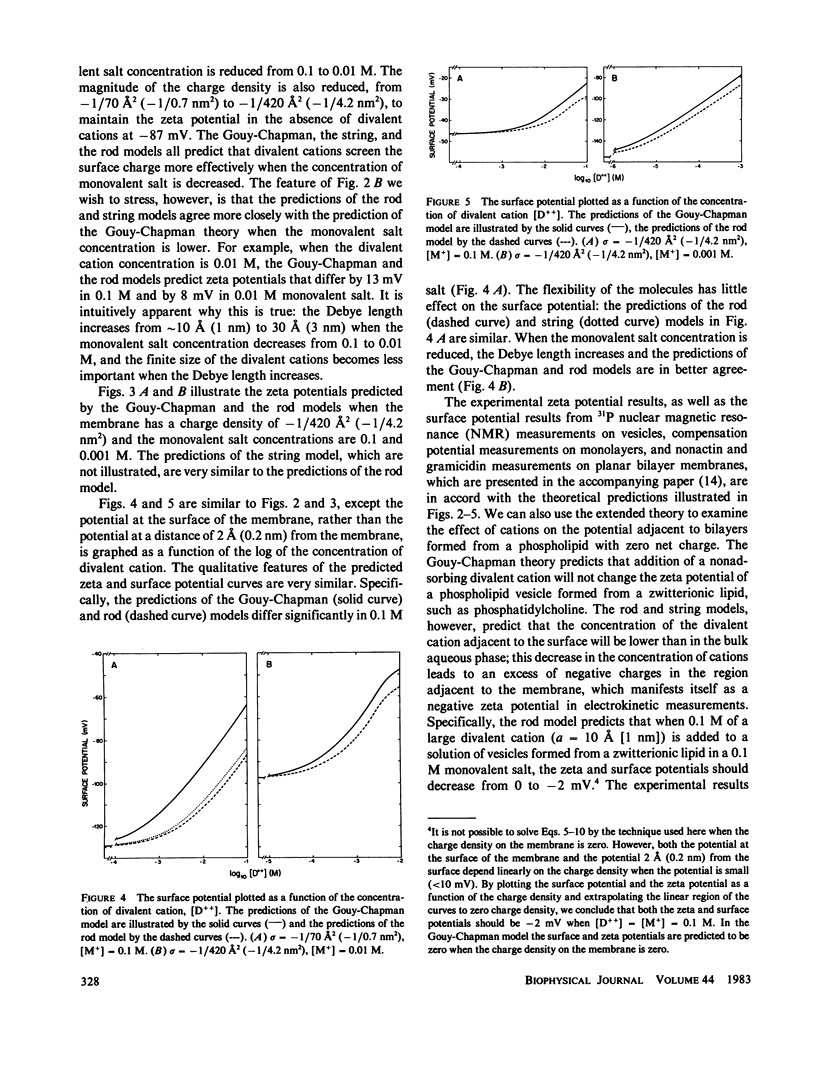
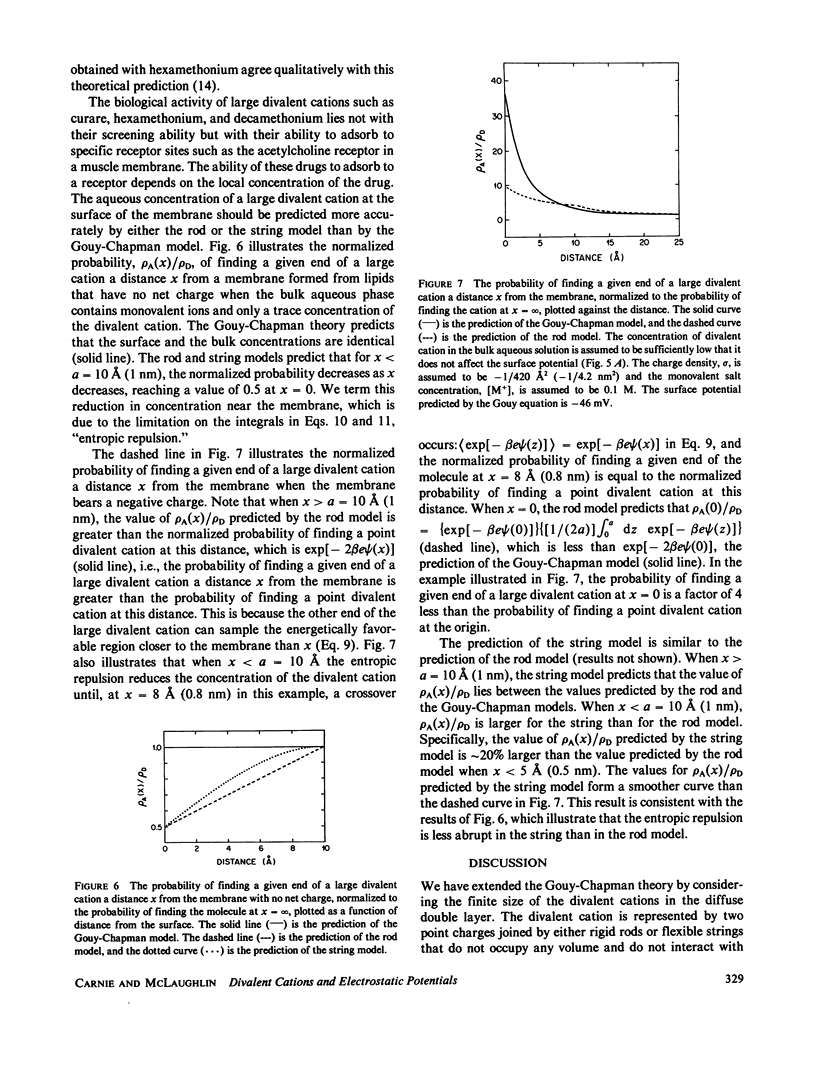
We have extended the Gouy-Chapman theory of the electrostatic diffuse double layer by considering the finite size of divalent cations in the aqueous phase adjacent to a charged surface. The divalent cations are modeled as either two point charges connected by an infinitely thin, rigid "rod" or two noninteracting point charges connected by an infinitely thin, flexible "string." We use the extended theory to predict the effects of a cation of length 10 A (1 nm) on the zeta and surface potentials of phospholipid bilayer membranes. The predictions of the rod and string models are similar to one another but differ markedly from the predictions of the Gouy-Chapman theory. Specifically, the extended model predicts that a large divalent cation will have a smaller effect on the potential adjacent to a negatively charged bilayer membrane than a point divalent cation, that the magnitude of this discrepancy will decrease as the Debye length increases, and that a large divalent cation will produce a negative zeta potential on a membrane formed from zwitterionic lipids. These predictions agree qualitatively with the experimental results obtained with the large divalent cation hexamethonium. We discuss the biological relevance of our calculations in the context of the interaction of cationic drugs with receptor sites on cell membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez O., Brodwick M., Latorre R., McLaughlin A., McLaughlin S., Szabo G. Large divalent cations and electrostatic potentials adjacent to membranes. Experimental results with hexamethonium. Biophys J. 1983 Dec;44(3):333–342. doi: 10.1016/S0006-3495(83)84307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boheim G., Hanke W., Barrantes F. J., Eibl H., Sakmann B., Fels G., Maelicke A. Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3586–3590. doi: 10.1073/pnas.78.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3936–3940. doi: 10.1073/pnas.71.10.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., McLaughlin A., McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta. 1981 Jul 20;645(2):279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. G., Cohen I. Membrane surface potential changes may alter drug interactions: an example, acetylcholine and curare. Science. 1979 Mar 30;203(4387):1351–1352. doi: 10.1126/science.424757. [DOI] [PubMed] [Google Scholar]


