Abstract
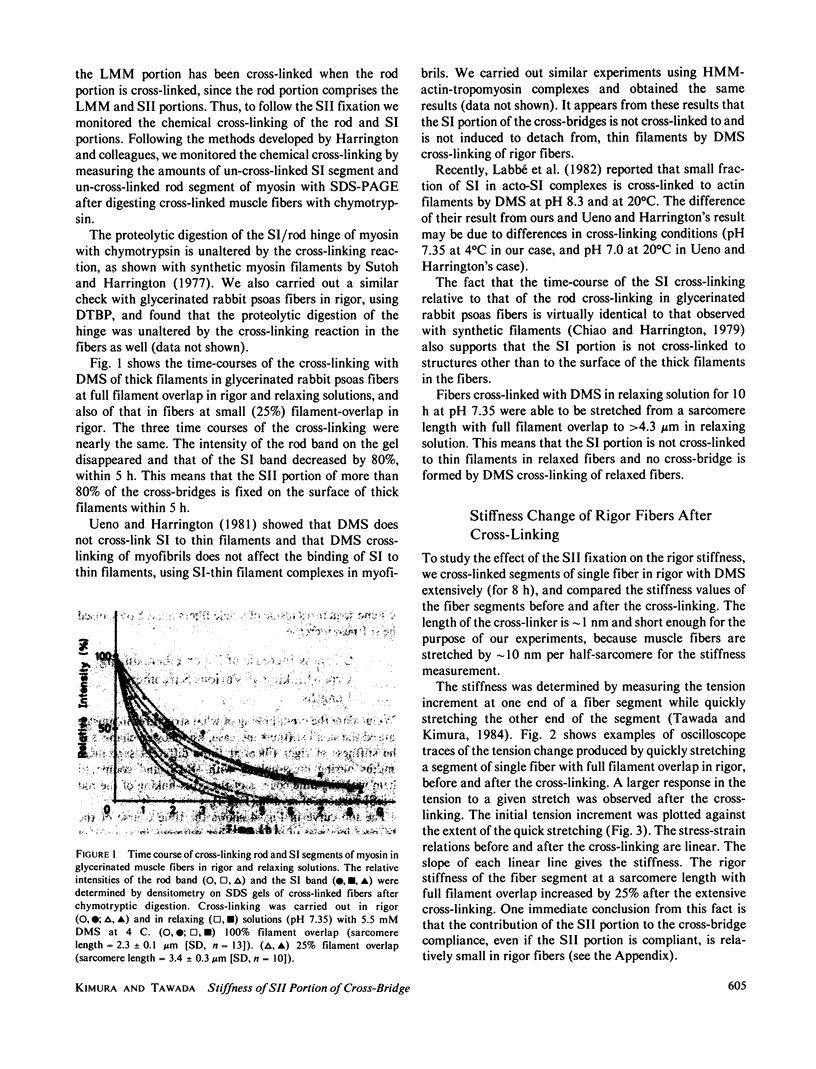
To see whether the SII portion of the cross-bridge in rigor fibers is longitudinally compliant, we chemically cross-linked with dimethyl suberimidate the entire rod portion (including the SII portion) of myosin onto the surface of thick filaments in glycerinated rabbit psoas fibers, and studied the effect of the SII fixation on the stiffness of the rigor fibers. The cross-linking of fiber segments with full filament overlap increased the rigor stiffness by approximately 25%. Almost the same absolute amount of the stiffness increase was also observed in rigor fibers with half- or no filament overlap after the cross-linking, and a similar but somewhat larger increment of stiffness was observed in fiber segments cross-linked in relaxing solution. These results indicate that the stiffness increase is not produced by the fixation of the SII portion onto the thick filament surface, but is caused instead by the cross-linking of some parallel elastic elements in muscle, and therefore indicate that the SII portion of the cross-bridge is hardly longitudinally compliant in rigor fibers.
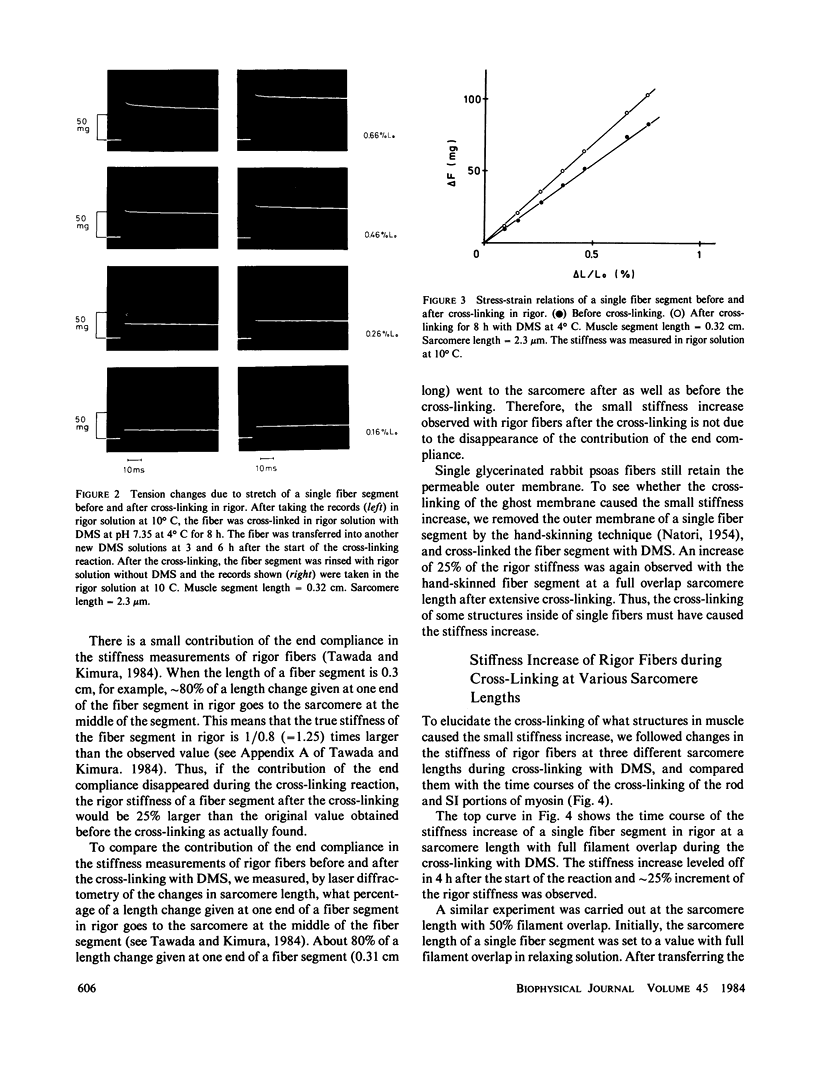
Full text
PDF

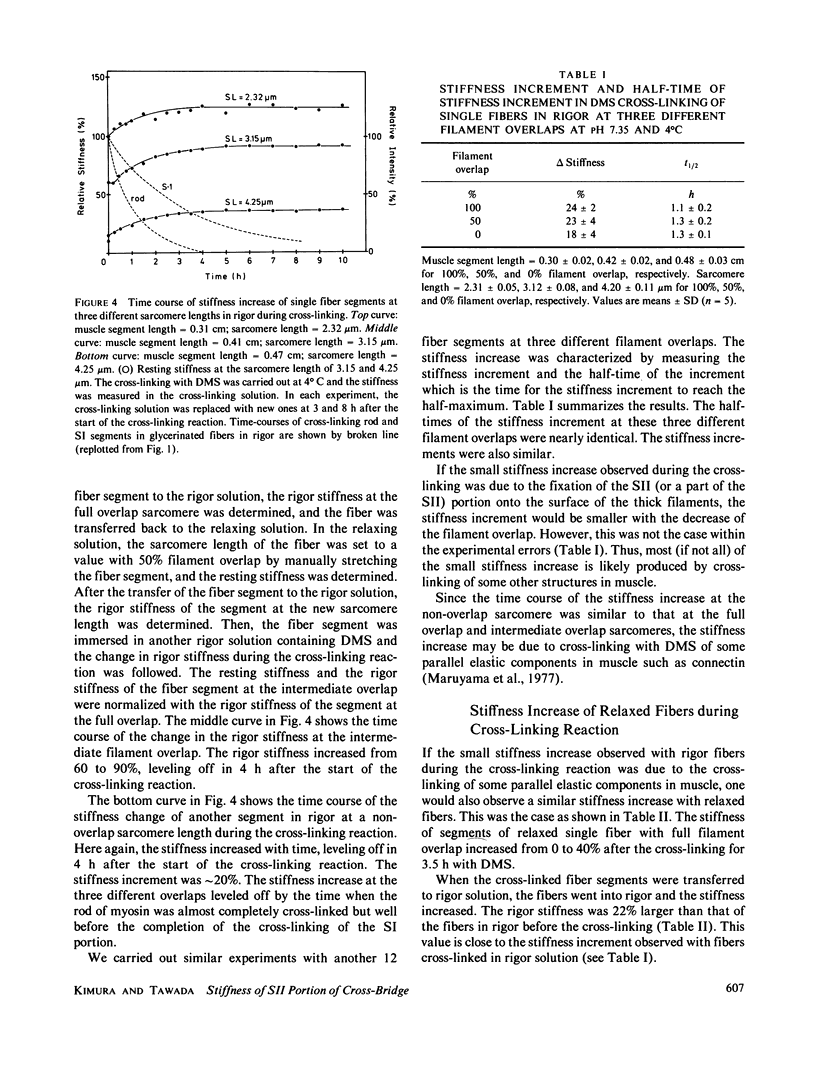



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao Y. C., Harrington W. F. Cross-bridge movement in glycerinated rabbit psoas muscle fibers. Biochemistry. 1979 Mar 20;18(6):959–963. doi: 10.1021/bi00573a004. [DOI] [PubMed] [Google Scholar]
- Cooke R. Stress does not alter the conformation of a domain of the myosin cross-bridge in rigor muscle fibres. Nature. 1981 Dec 10;294(5841):570–571. doi: 10.1038/294570a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. A cross-bridge model of muscle contraction. Prog Biophys Mol Biol. 1978;33(1):55–82. doi: 10.1016/0079-6107(79)90025-7. [DOI] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth K., Kuhn H. J. Stiffness and tension during and after sudden length changes of glycerinated rabbit psoas muscle fibres. Biophys Struct Mech. 1978 Jul 12;4(3):223–236. doi: 10.1007/BF02426087. [DOI] [PubMed] [Google Scholar]
- Harrington W. F. A mechanochemical mechanism for muscle contraction. Proc Natl Acad Sci U S A. 1971 Mar;68(3):685–689. doi: 10.1073/pnas.68.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F. On the origin of the contractile force in skeletal muscle. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5066–5070. doi: 10.1073/pnas.76.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Kretzschmar K. M., O'Konski C. T., Morales M. F. Flexibility of myosin rod, light meromyosin, and myosin subfragment-2 in solution. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4986–4990. doi: 10.1073/pnas.74.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Hvidt S., Nestler F. H., Greaser M. L., Ferry J. D. Flexibility of myosin rod determined from dilute solution viscoelastic measurements. Biochemistry. 1982 Aug 17;21(17):4064–4073. doi: 10.1021/bi00260a024. [DOI] [PubMed] [Google Scholar]
- Labbé J. P., Mornet D., Roseau G., Kassab R. Cross-linking of F-actin to skeletal muscle myosin subfragment 1 with bis(imido esters): further evidence for the interaction of myosin-head heavy chain with an actin dimer. Biochemistry. 1982 Dec 21;21(26):6897–6902. doi: 10.1021/bi00269a042. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Matsubara S., Natori R., Nonomura Y., Kimura S. Connectin, an elastic protein of muscle. Characterization and Function. J Biochem. 1977 Aug;82(2):317–337. [PubMed] [Google Scholar]
- Naylor G. R., Podolsky R. J. X-ray diffraction of strained muscle fibers in rigor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5559–5563. doi: 10.1073/pnas.78.9.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. An actin-binding site on the 20K fragment of myosin subfragment 1. Biochemistry. 1982 Sep 14;21(19):4800–4804. doi: 10.1021/bi00262a043. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Harrington W. F. Cross-linking of myosin thick filaments under activating and rigor conditions. A study of the radial disposition of cross-bridges. Biochemistry. 1977 May 31;16(11):2441–2449. doi: 10.1021/bi00630a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Location of SH1 and SH2 along a heavy chain of myosin subfragment 1. Biochemistry. 1981 May 26;20(11):3281–3285. doi: 10.1021/bi00514a046. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983 Mar 29;22(7):1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- Tawada Y., Oara H., Ooi T., Tawada K. Non-polymerizable tropomyosin and control of the superprecipitation of actomyosin. J Biochem. 1975 Jul;78(1):65–72. [PubMed] [Google Scholar]
- Tsong T. Y., Karr T., Harrington W. F. Rapid helix--coil transitions in the S-2 region of myosin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1109–1113. doi: 10.1073/pnas.76.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Harrington W. F. Cross-bridge movement and the conformational state of the myosin hinge in skeletal muscle. J Mol Biol. 1981 Jul 15;149(4):619–640. doi: 10.1016/0022-2836(81)90350-8. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Herzig J. W. Series elastic properties of skinned muscle fibres in contraction and rigor. Pflugers Arch. 1978 Jan 31;373(1):21–24. doi: 10.1007/BF00581144. [DOI] [PubMed] [Google Scholar]



