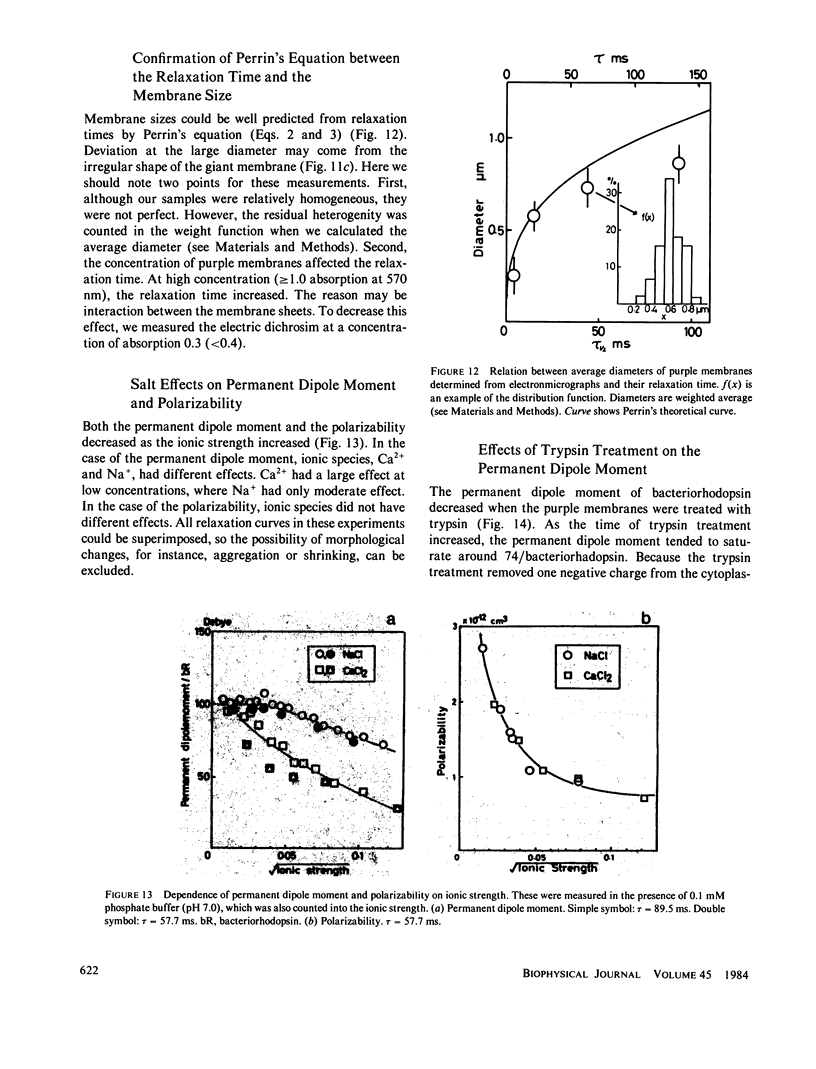
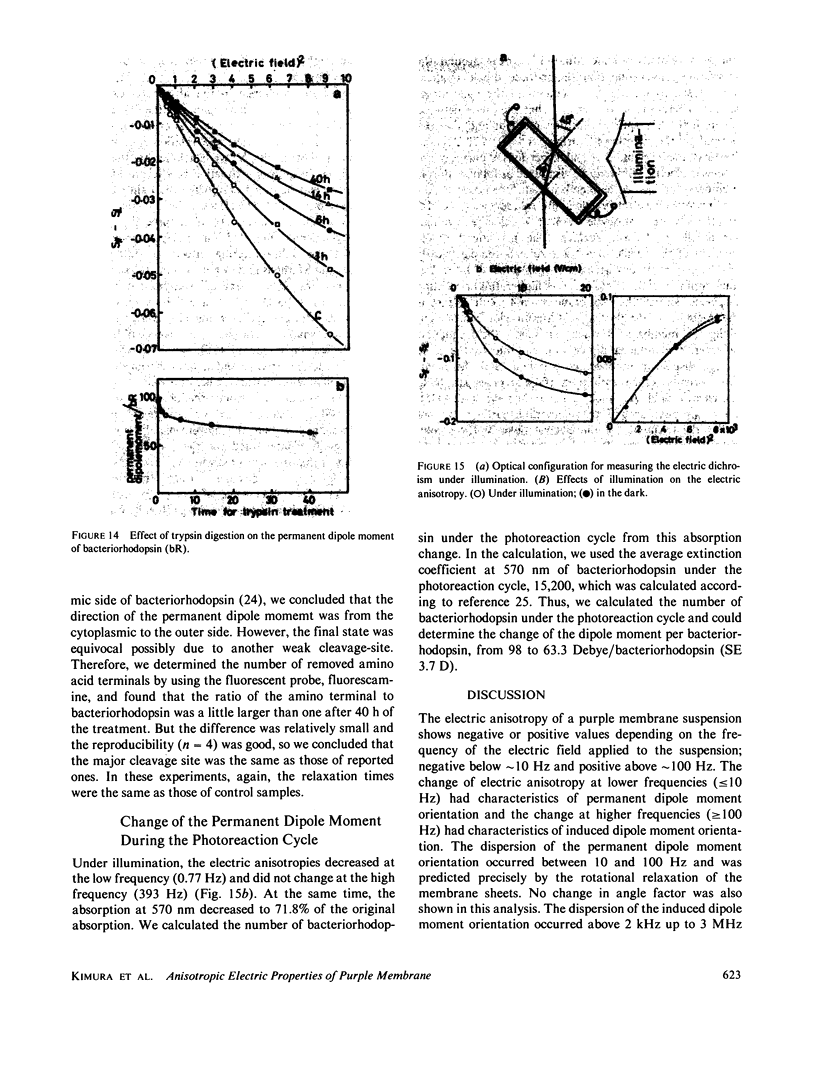
Abstract
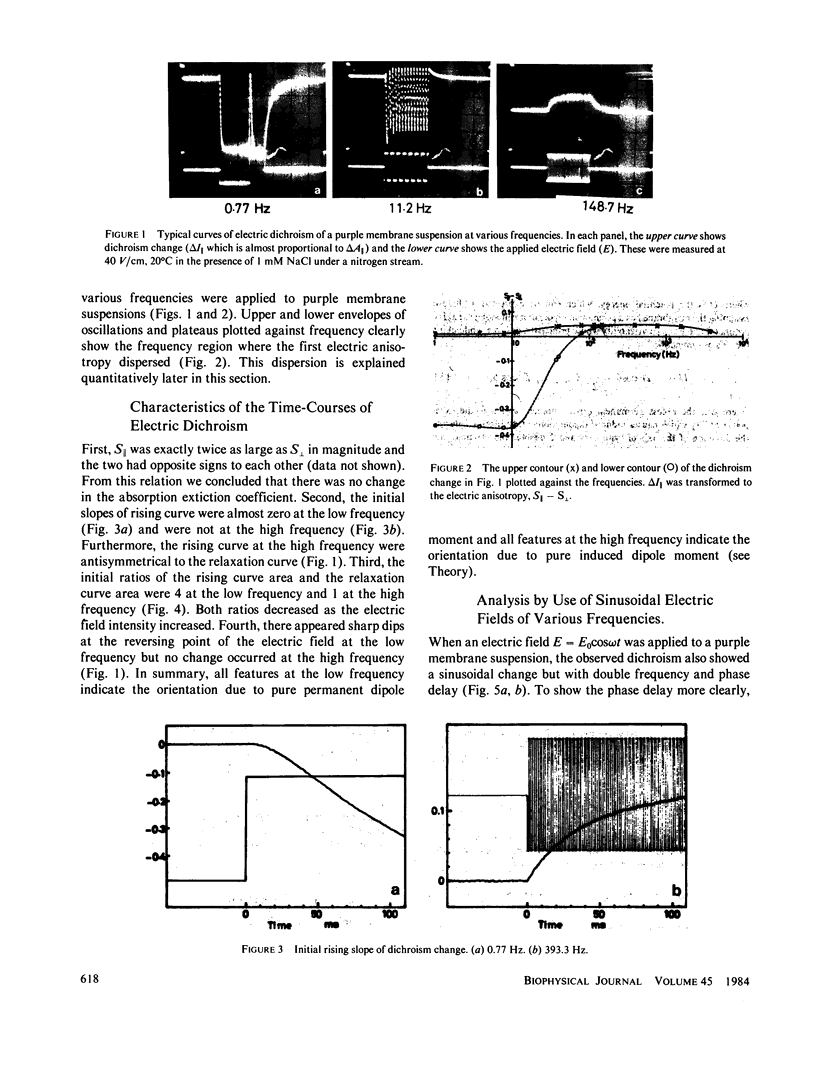
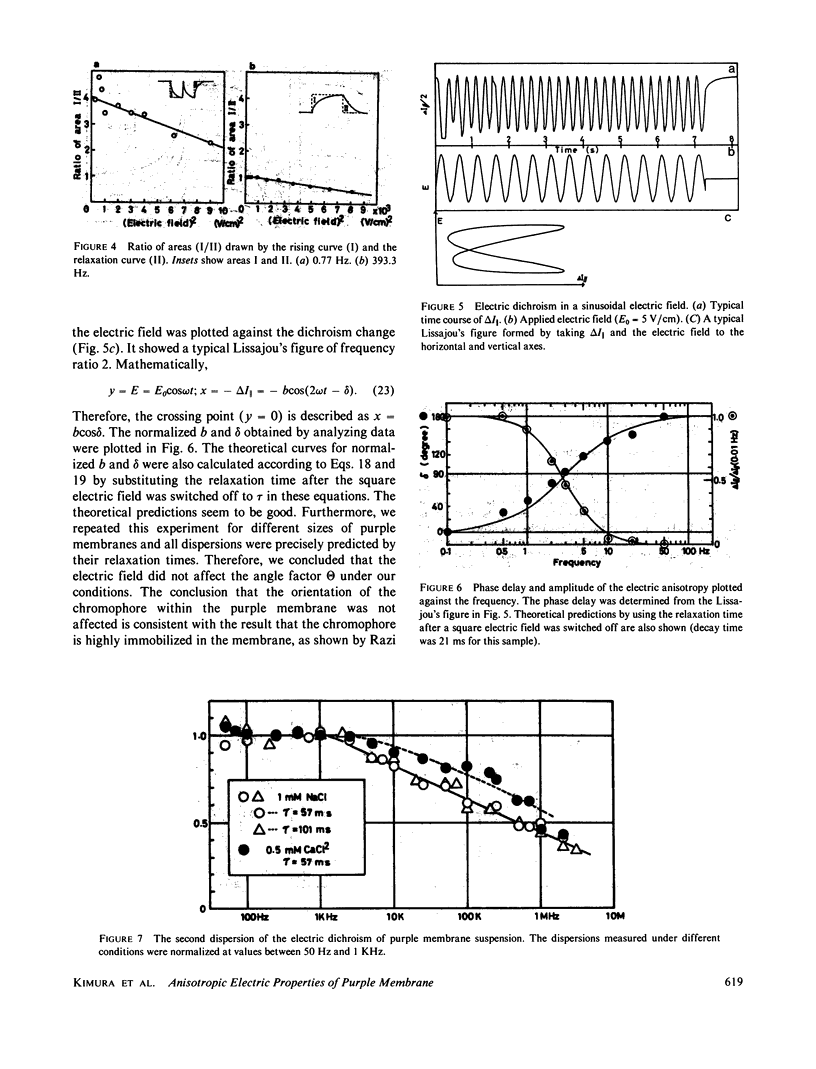
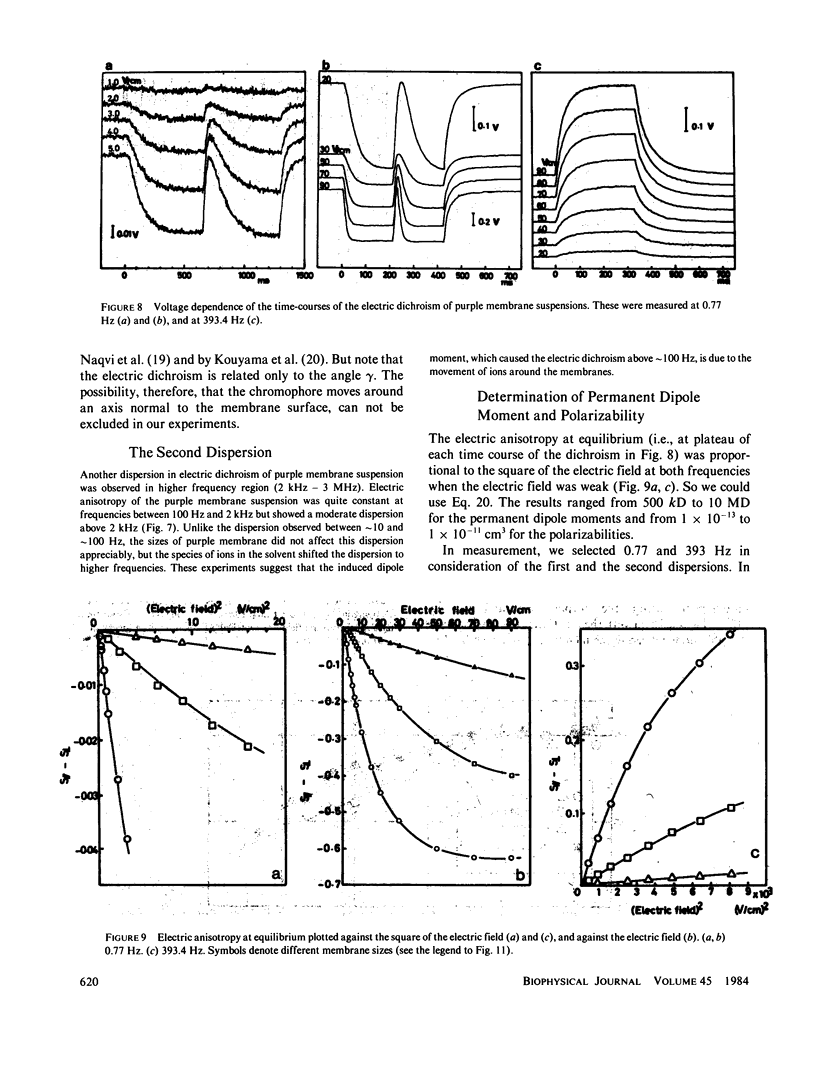
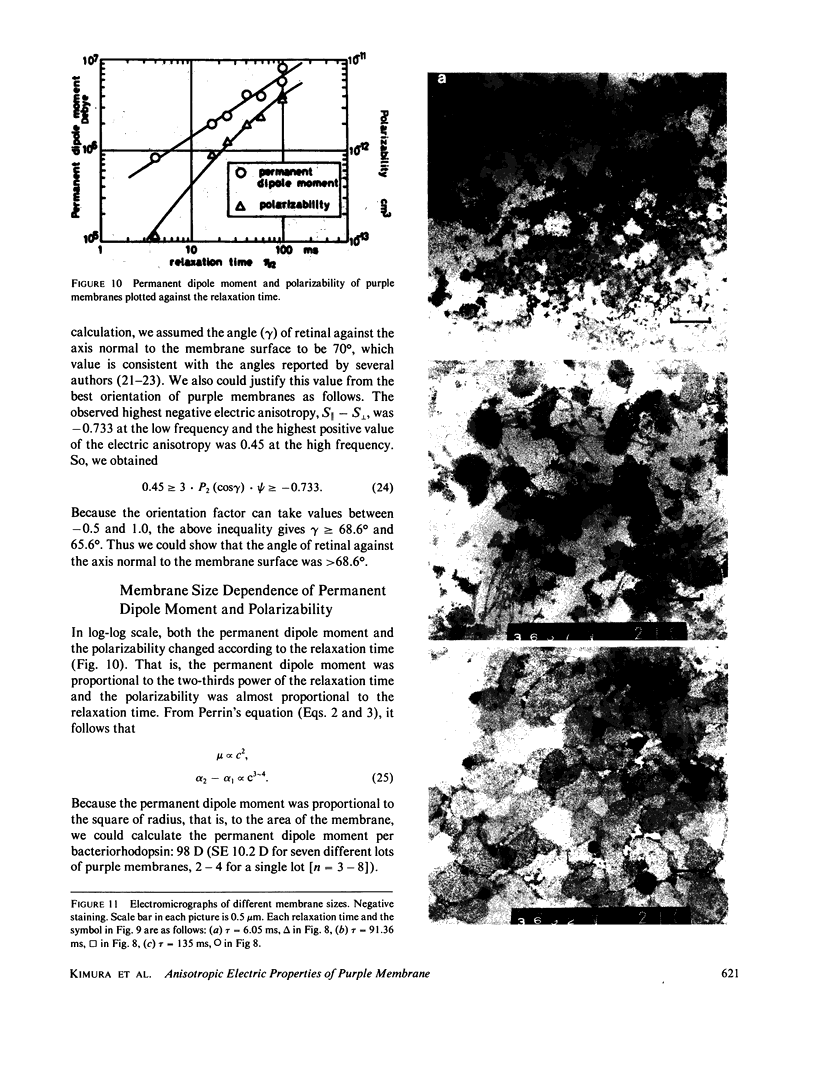
Purple membrane suspension shows two different orientations in electric fields of different frequencies. The orientation at low frequencies (less than or equal to approximately 10 Hz), with the membrane surface perpendicular to the electric field, is due to permanent dipole moment of the membrane and the orientation at high frequencies (greater than or equal to approximately 100 Hz), with the surface parallel to the electric field, is due to induced dipole moment. By quantitative analysis of these orientations, we determined the permanent dipole moment and the polarizability. Both values varied according to the membrane size: the permanent dipole moment ranged from 500 kD to 10 MD and was proportional to the square of the diameter of the membrane. The polarizability ranged from 1 X 10(-13) to 1 X 10(-11)cm3 and was proportional to the third to fourth power of the diameter. Because the permanent dipole moment was proportional to the area of the membrane, we could determine permanent dipole moment per bacteriorhodopsin. By determining the actual membrane size under electron microscopy, we got 98 D/bacteriorhodopsin. We also concluded that the direction of the permanent dipole moment was from the cytoplasmic to the extracellular side. These values, however, were strongly dependent on the ionic strength in the medium, suggesting a screening effect due to counter ions near the membrane surface. We evaluated the screening effect and showed about a four-charge difference between the two sides of the purple membrane. Under illumination, we found that the permanent dipole moment decreased from 98 to 63 D/bacteriorhodopsin. From the best-oriented sample, we also concluded that the angle of retinal against the axis normal to the membrane surface was greater than 68.6 degrees.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Druckmann S., Ottolenghi M. Electric dichroism in the purple membrane of Halobacterium halobium. Biophys J. 1981 Feb;33(2):263–268. doi: 10.1016/S0006-3495(81)84887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G. E., Gray C. P., Wildenauer D., Khorana H. G. Orientation of bacteriorhodopsin in Halobacterium halobium as studied by selective proteolysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5426–5430. doi: 10.1073/pnas.74.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Keszthelyi L. Orientation of membrane fragments by electric field. Biochim Biophys Acta. 1980 Jun 6;598(3):429–436. doi: 10.1016/0005-2736(80)90023-1. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Immobilization of bacteriorhodopsin and orientation of its transition moment in purple membrane. FEBS Lett. 1978 May 1;89(1):15–20. doi: 10.1016/0014-5793(78)80512-2. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Petersen D. C., Cone R. A. The electric dipole moment of rhodopsin solubilized in Triton X-100. Biophys J. 1975 Dec;15(12):1181–1200. doi: 10.1016/S0006-3495(75)85894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi Naqvi K., Gonzalez-Rodriguez J., Cherry R. J., Chapman D. Spectroscopic technique for studying protein rotation in membranes. Nat New Biol. 1973 Oct 24;245(147):249–251. doi: 10.1038/newbio245249a0. [DOI] [PubMed] [Google Scholar]
- Shinar R., Druckmann S., Ottolenghi M., Korenstein R. Electric field effects in bacteriorhodopsin. Biophys J. 1977 Jul;19(1):1–5. doi: 10.1016/S0006-3495(77)85558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]





