Abstract
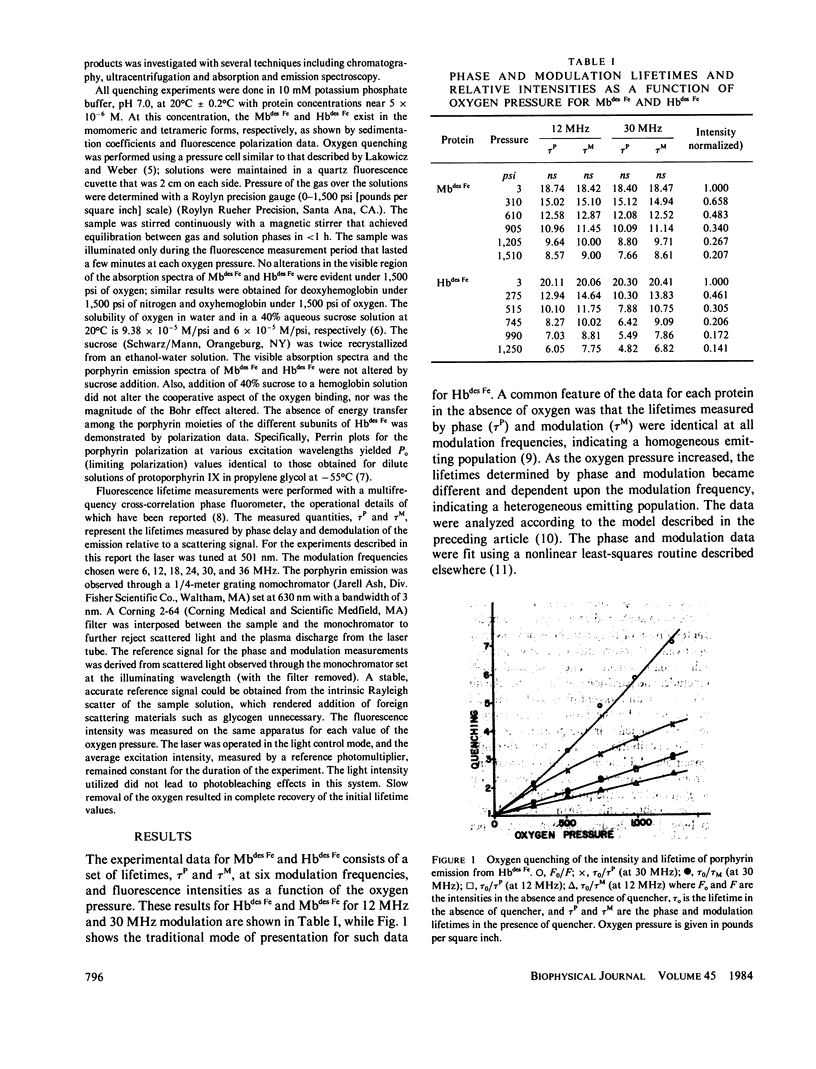
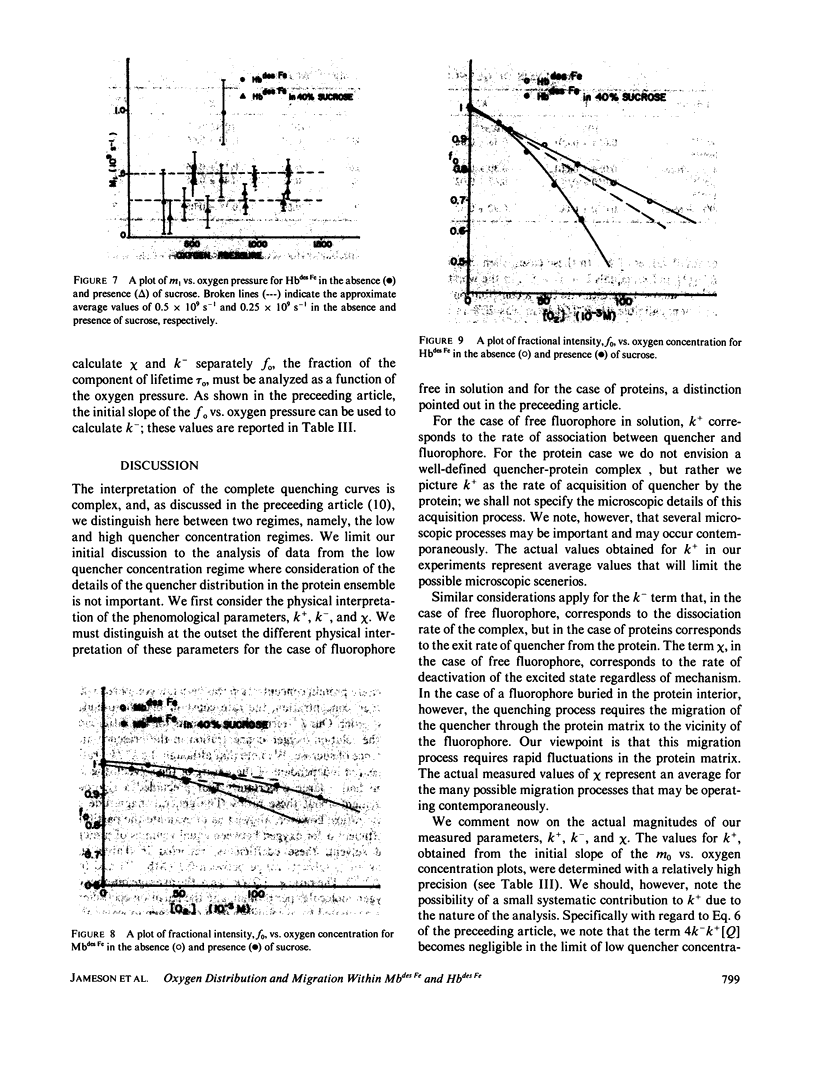
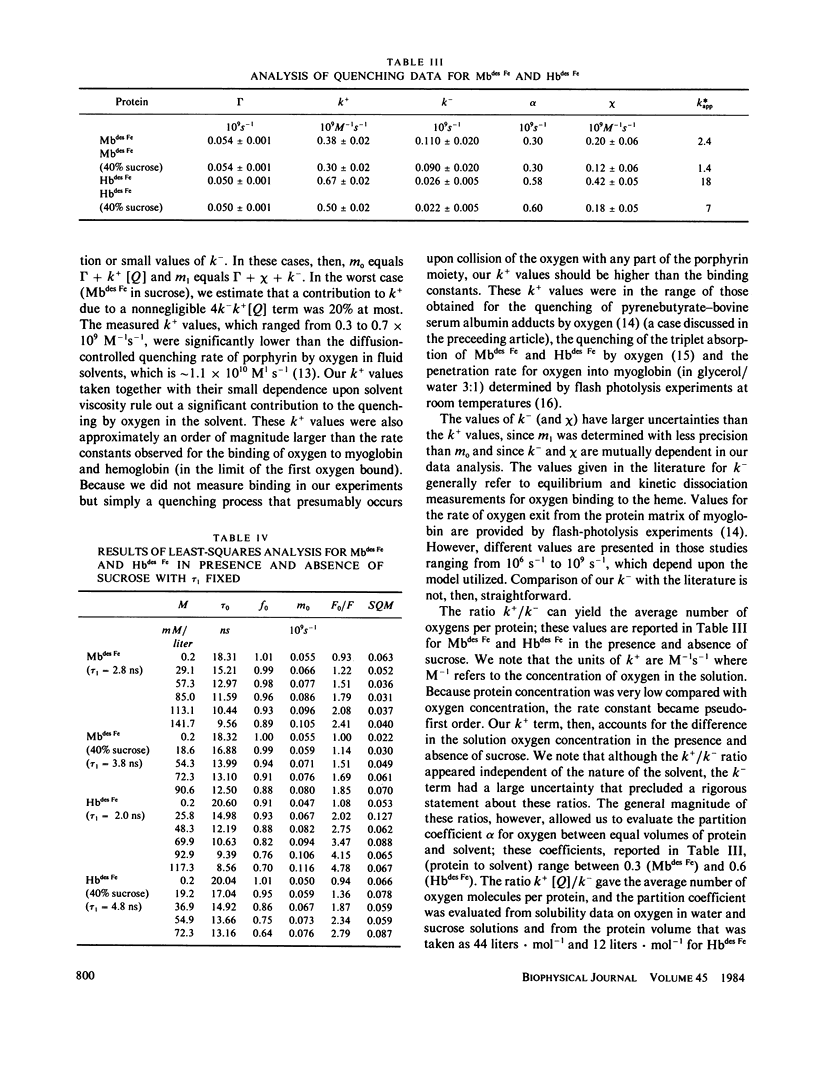
Quenching of the intensity and lifetime of porphyrin fluorescence from Mbdes Fe and Hbdes Fe (iron-free myoglobin and hemoglobin) by oxygen was investigated using a multifrequency cross-correlation phase fluorometer. The single exponential decay characteristic of porphyrin emission of Mbdes Fe and Hbdes Fe became doubly exponential upon application of oxygen pressure. The results were interpreted in terms of a general model of dynamic quenching of fluorescence in globular proteins. The model accounted for the rate k+ of acquisition of quencher by the protein, the exit rate k- of quencher from the protein, and the migration rate chi of quencher in the protein interior. The values of k+, k-, and chi were different for Mbdes Fe and Hbdes Fe. The addition of 40% sucrose, which increased the bulk viscosity sixfold, modified these rates. These results are discussed and compared with previous quenching studies on proteins. The significance of these results and the model for the interpretation of protein quenching studies is emphasized.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Coppey M., Jameson D. M., Alpert B. Oxygen diffusion through hemoglobin and HbdesFe: quenching of the tryptophan and porphyrin emissions. FEBS Lett. 1981 Apr 20;126(2):191–194. doi: 10.1016/0014-5793(81)80239-6. [DOI] [PubMed] [Google Scholar]
- Doster W. Viscosity scaling and protein dynamics. Biophys Chem. 1983 Mar;17(2):97–103. doi: 10.1016/0301-4622(83)80002-7. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Fluorescence quenching studies with proteins. Anal Biochem. 1981 Jul 1;114(2):199–227. doi: 10.1016/0003-2697(81)90474-7. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Weber G., Alpert B. A model of dynamic quenching of fluorescence in globular proteins. Biophys J. 1984 Apr;45(4):789–794. doi: 10.1016/S0006-3495(84)84223-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton E., Limkeman M. A continuously variable frequency cross-correlation phase fluorometer with picosecond resolution. Biophys J. 1983 Dec;44(3):315–324. doi: 10.1016/S0006-3495(83)84305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry. 1973 Oct 9;12(21):4161–4170. doi: 10.1021/bi00745a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebban P., Coppey M., Alpert B., Lindqvist L., Jameson D. M. Fluorescence properties of porphyrin-globin from human hemoglobin. Photochem Photobiol. 1980 Dec;32(6):727–731. doi: 10.1111/j.1751-1097.1980.tb04049.x. [DOI] [PubMed] [Google Scholar]
- Tilton R. F., Jr, Kuntz I. D., Jr Nuclear magnetic resonance studies of xenon-129 with myoglobin and hemoglobin. Biochemistry. 1982 Dec 21;21(26):6850–6857. doi: 10.1021/bi00269a035. [DOI] [PubMed] [Google Scholar]
- Vaughan W. M., Weber G. Oxygen quenching of pyrenebutyric acid fluorescence in water. A dynamic probe of the microenvironment. Biochemistry. 1970 Feb 3;9(3):464–473. doi: 10.1021/bi00805a003. [DOI] [PubMed] [Google Scholar]


