Abstract
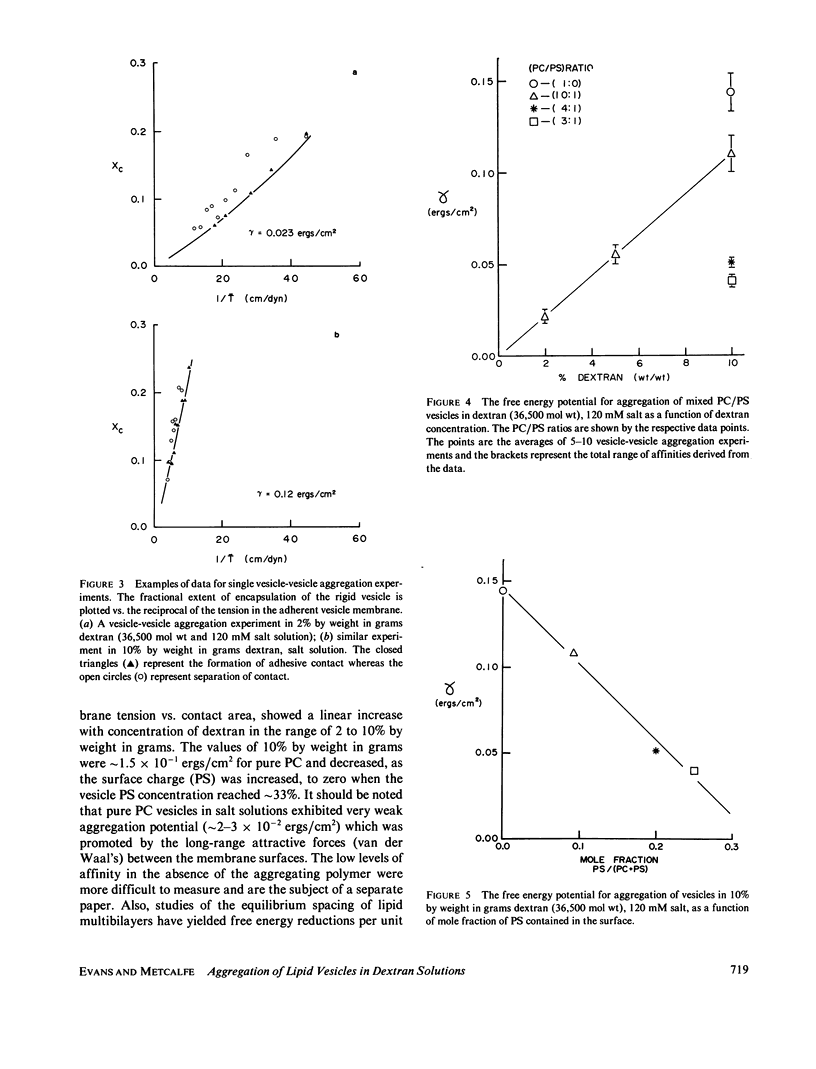
The energetics of lipid vesicle-vesicle aggregation in dextran (36,000 mol wt) solutions have been studied with the use of micromechanical experiments. The affinities (free energy reduction per unit area of contact) for vesicle-vesicle aggregation were determined from measurements of the tension induced in an initially flaccid vesicle membrane as it adhered to another vesicle. The experiments involved controlled aggregation of single vesicles by the following procedure: two giant (approximately 20 micron diam) vesicles were selected from a chamber on the microscope stage that contained the vesicle suspension and transferred to a second chamber that contained a dextran (36,000 mol wt) salt solution (120 mM); the vesicles were then maneuvered into position for contact. One vesicle was aspirated with sufficient suction pressure to create a rigid sphere outside the pipette; the other vesicle was allowed to spread over the rigid vesicle surface. The aggregation potential (affinity) was derived from the membrane tension vs. contact area. Vesicles were formed from mixture of egg lecithin (PC) and phosphatidylserine (PS). For vesicles with a PC/PS ratio of 10:1, the affinity showed a linear increase with concentration of dextran; the values were on the order of 10(-1) ergs/cm2 at 10% by weight in grams. Similarly, pure PC vesicle aggregation was characterized by an affinity value of 1.5 X 10(-1) ergs/cm2 in 10% dextran by weight in grams. In 10% by weight in grams solutions of dextran, the free energy potential for vesicle aggregation decreased as the surface charge (PS) was increased; the affinity extrapolated to zero at a PC/PS ratio of 2:1. When adherent vesicle pairs were transferred into a dextran-free buffer, the vesicles did not spontaneously separate. They maintained adhesive contact until forceably separated, after which they would not read here. Thus, it appears that dextran forms a "cross-bridge" between the vesicle surfaces.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxbaum K., Evans E., Brooks D. E. Quantitation of surface affinities of red blood cells in dextran solutions and plasma. Biochemistry. 1982 Jun 22;21(13):3235–3239. doi: 10.1021/bi00256a032. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Analysis of adhesion of large vesicles to surfaces. Biophys J. 1980 Sep;31(3):425–431. doi: 10.1016/S0006-3495(80)85069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Kukan B. Free energy potential for aggregation of erythrocytes and phosphatidylcholine/phosphatidylserine vesicles in Dextran (36,500 MW) solutions and in plasma. Biophys J. 1983 Nov;44(2):255–260. doi: 10.1016/S0006-3495(83)84297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan K. M., Chien S. Role of surface electric charge in red blood cell interactions. J Gen Physiol. 1973 May;61(5):638–654. doi: 10.1085/jgp.61.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosley-Millman M. E., Rand R. P., Parsegian V. A. Effects of monovalent ion binding and screening on measured electrostatic forces between charged phospholipid bilayers. Biophys J. 1982 Dec;40(3):221–232. doi: 10.1016/S0006-3495(82)84477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Papahadjopoulos D. Calcium/magnesium specificity in membrane fusion: kinetics of aggregation and fusion of phosphatidylserine vesicles and the role of bilayer curvature. Biochemistry. 1981 May 26;20(11):3126–3133. doi: 10.1021/bi00514a022. [DOI] [PubMed] [Google Scholar]
- Wong M., Thompson T. E. Aggregation of dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1982 Aug 17;21(17):4133–4139. doi: 10.1021/bi00260a033. [DOI] [PubMed] [Google Scholar]
- Wu P. S., Tin G. W., Baldeschwieler J. D., Shen T. Y., Ponpipom M. M. Effect of surface modification on aggregation of phospholipid vesicles. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6211–6215. doi: 10.1073/pnas.78.10.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]



