Abstract
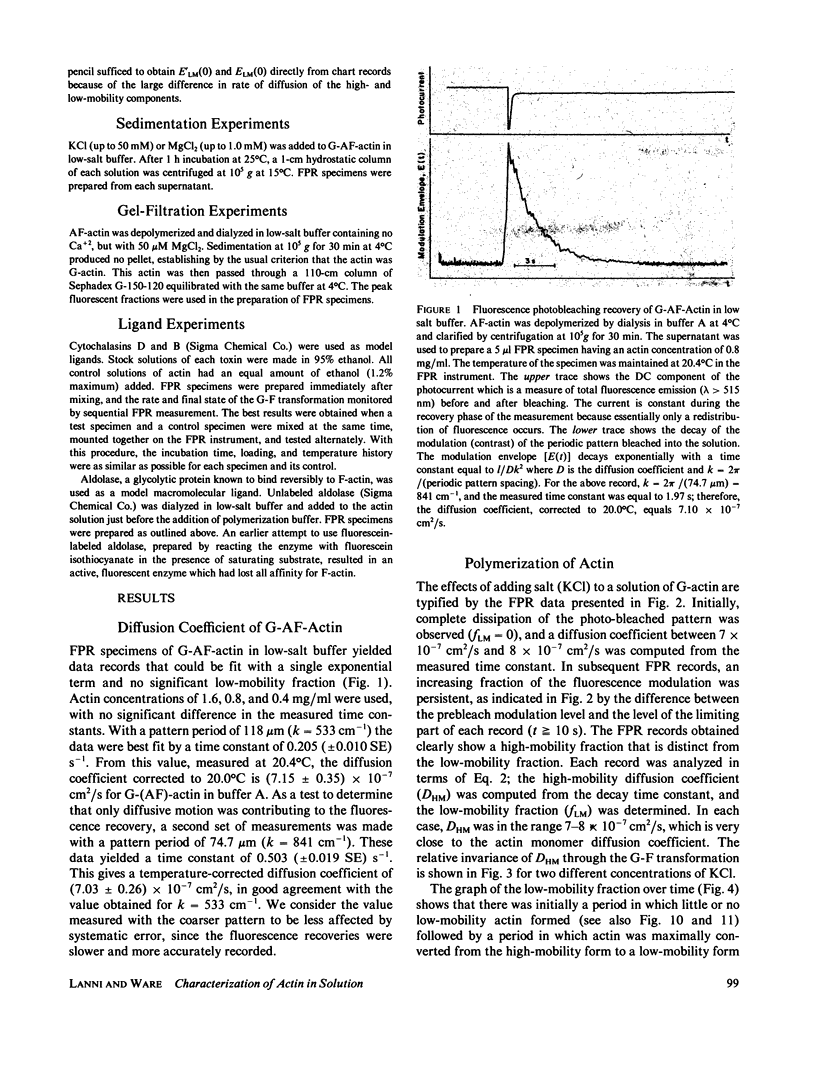
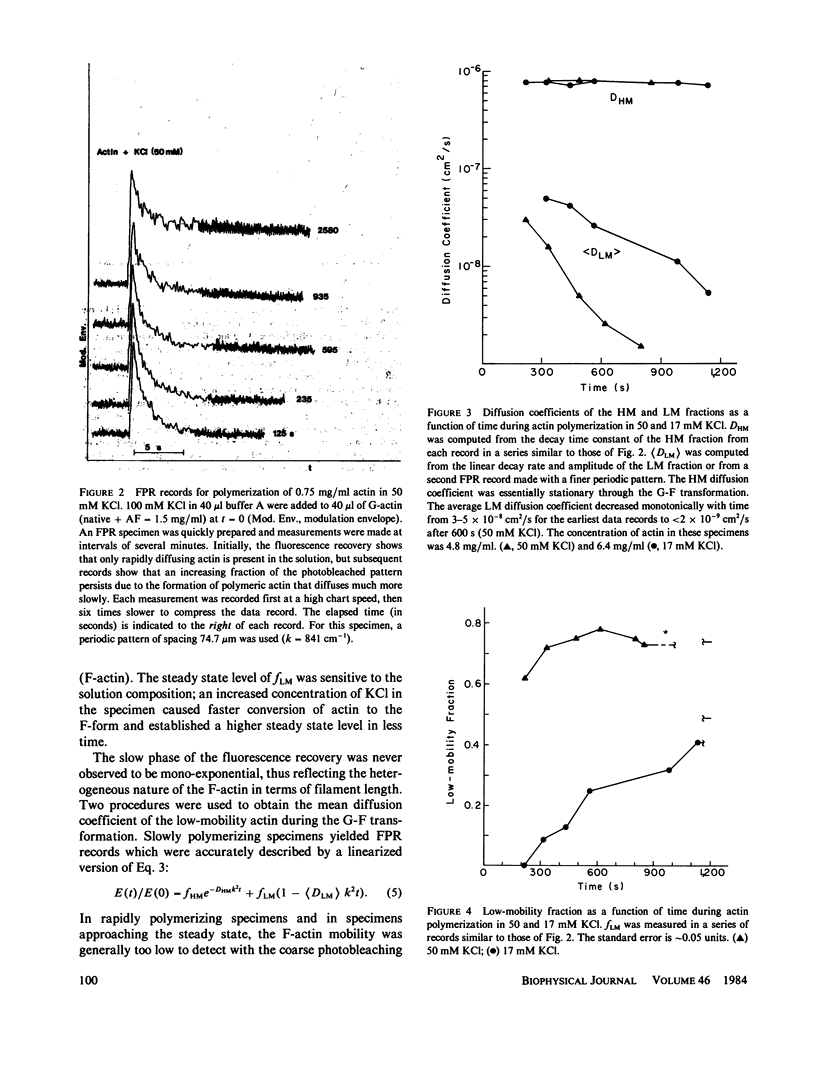
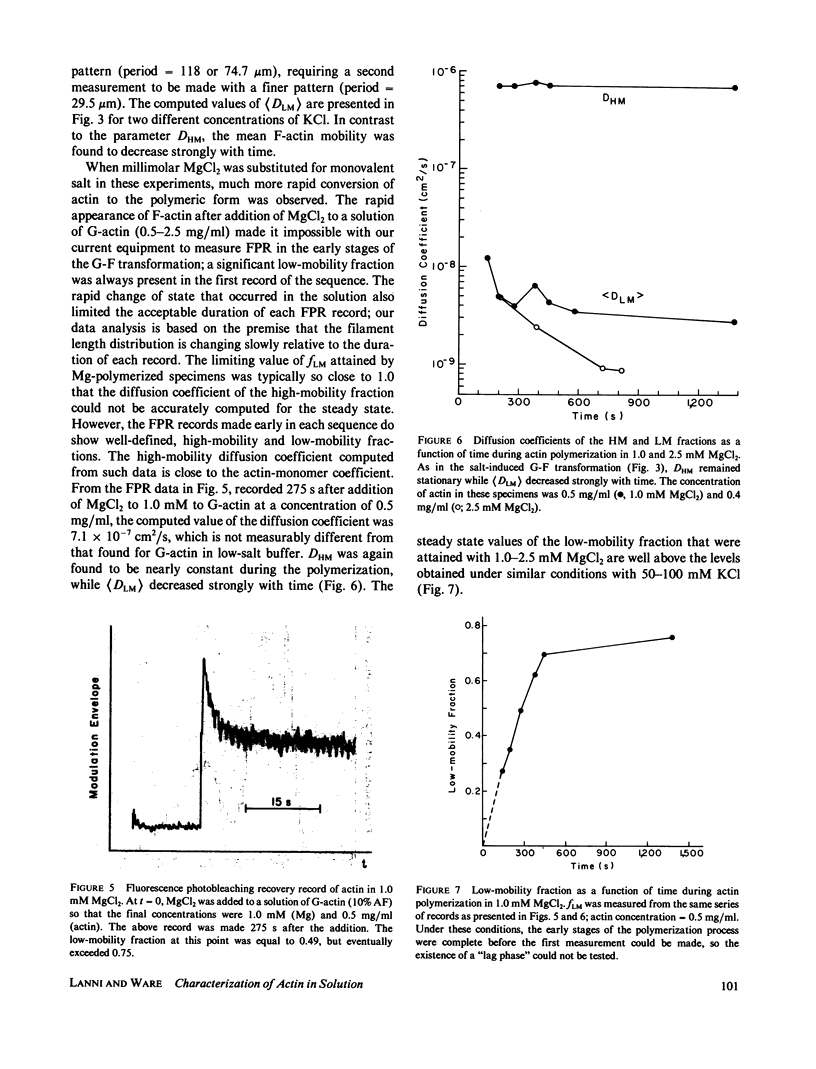
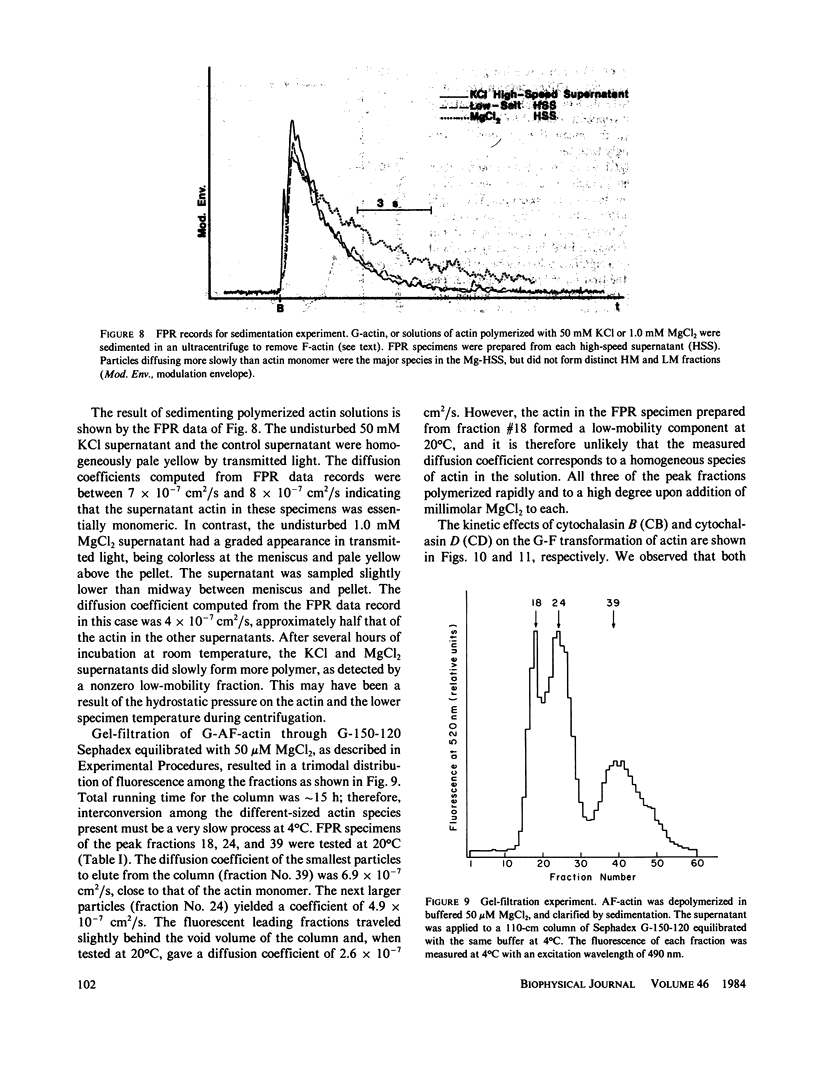
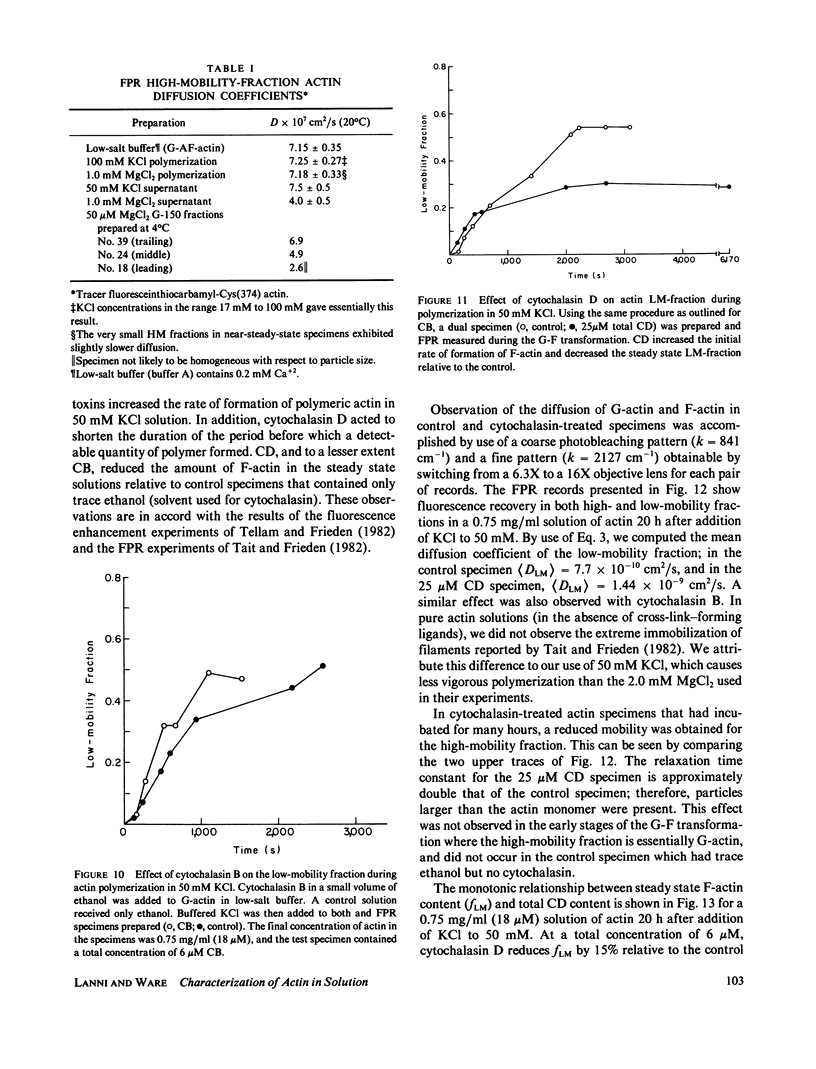
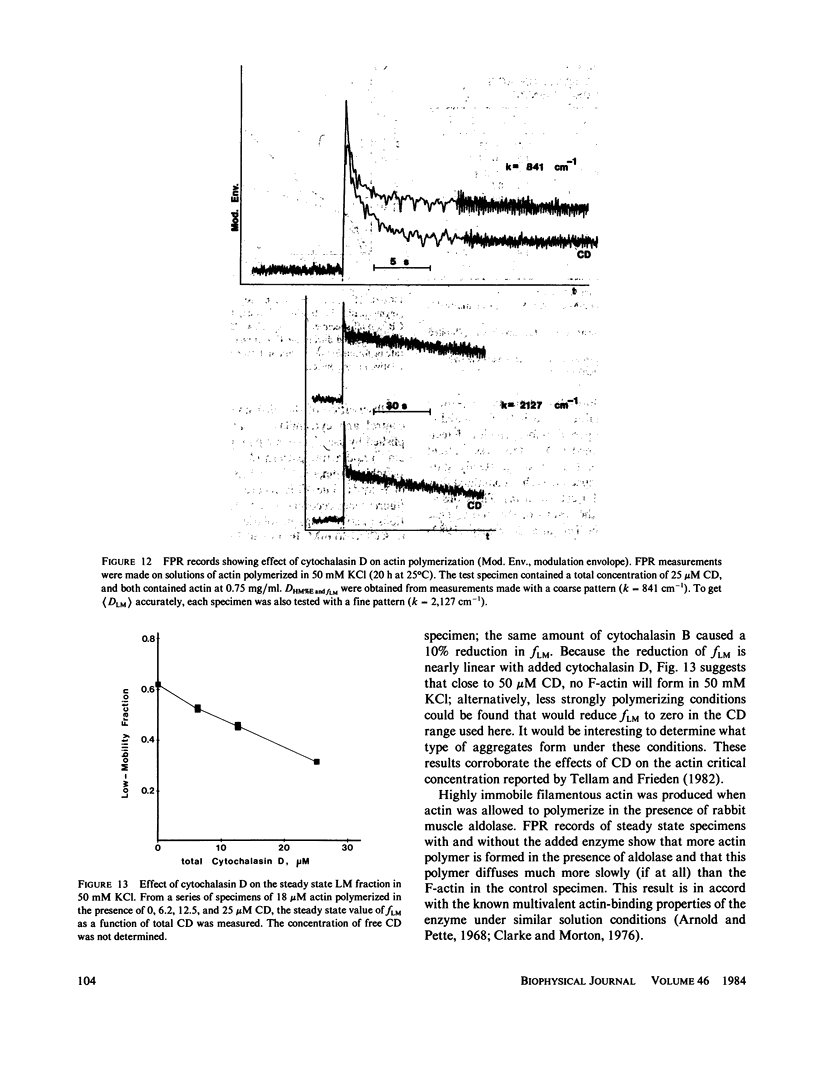
Fluorescence photobleaching recovery (FPR) was measured to determine the diffusion coefficient of fluorescein-labeled G-actin in low-salt buffer. The result obtained, 7.15 +/- 0.35 X 10(-7) cm2/s, is in good agreement with that computed from the molecular weight, partial specific volume, and sedimentation coefficient, but is higher than previously obtained values. It is demonstrated from theory that at low ionic strength, the electrostatic contribution to the intrinsic viscosity leads to an overestimate of the hydrodynamic eccentricity of G-actin. Data from FPR, sedimentation, and fluorescence polarization experiments all indicate that the true low-salt form of the actin monomer has an axial ratio less than or equal to 3.0. The G-F transformation of actin was also observed by measurement of FPR during the assembly phase, in the steady state, and in the presence of ligands such as cytochalasin and aldolase. Each FPR record in general yields three data: relative proportion of rapidly and slowly diffusing actin, diffusion coefficient for the high-mobility fraction, and a mean diffusion coefficient for the low-mobility fraction. A relation between the mean low-mobility diffusion coefficient and the number-average filament length is derived and applied to the analysis of FPR data. Under typical conditions, the average filament length was much greater than 10 micron in the steady state. Cytochalasin D was found to decrease filament length and total amount of filament proportionally; total filament number was not greatly affected. In all polymerizations of G-actin, the high-mobility material observed in situ was found to be essentially monomeric actin. Relatively stable oligomers of actin were separated by fractionating G-AF-actin by gel filtration in 50 microM MgCl2 at 4 degrees C. On the basis of the diffusion coefficient, we conclude that monomer and dimer constitute the major particle types present under these conditions. Sedimentation of labeled actin polymerized in 1.0 mM MgCl2 yielded a graded supernatant that contained actin oligomers significantly larger than the monomer.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASAKURA S., OOSAWA F. Dephosphorylation of adenosine triphosphate in actin solutions at low concentrations of magnesium. Arch Biochem Biophys. 1960 Apr;87:273–280. doi: 10.1016/0003-9861(60)90172-7. [DOI] [PubMed] [Google Scholar]
- Aebi U., Smith P. R., Isenberg G., Pollard T. D. Structure of crystalline actin sheets. Nature. 1980 Nov 20;288(5788):296–298. doi: 10.1038/288296a0. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968 Nov;6(2):163–171. doi: 10.1111/j.1432-1033.1968.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H. C., Cooke R., Smith L. The G-actin is greater than F-actin transformation as studied by the fluorescence of bound dansyl cystine. Arch Biochem Biophys. 1971 Jan;142(1):333–339. doi: 10.1016/0003-9861(71)90291-8. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Morton D. J. Aldolase binding to actin-containing filaments. Formation of paracrystals. Biochem J. 1976 Dec 1;159(3):797–798. doi: 10.1042/bj1590797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Yang Y. Z., Korn E. D. Polymerization of Acanthamoeba actin. Kinetics, thermodynamics, and co-polymerization with muscle actin. J Biol Chem. 1976 Dec 10;251(23):7474–7479. [PubMed] [Google Scholar]
- Ikkai T., Wahl P., Auchet J. C. Anisotropy decay of labelled actin. Evidence of the flexibility of the peptide chain in F-actin molecules. Eur J Biochem. 1979 Jan 15;93(2):397–408. doi: 10.1111/j.1432-1033.1979.tb12836.x. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Aebi U., Pollard T. D. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980 Dec 4;288(5790):455–459. doi: 10.1038/288455a0. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The G-F equilibrium in actin solutions under various conditions. Biochim Biophys Acta. 1962 Feb 12;57:13–21. doi: 10.1016/0006-3002(62)91072-7. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The cooperative nature of G-F transformation of actin. Biochim Biophys Acta. 1962 Feb 12;57:22–31. doi: 10.1016/0006-3002(62)91073-9. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Electron microscopic particle length of F-actin polymerized in vitro. J Biochem. 1970 Mar;67(3):437–457. doi: 10.1093/oxfordjournals.jbchem.a129267. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982 Jul;29(3):835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lanni F., Taylor D. L., Ware B. R. Fluorescence photobleaching recovery in solutions of labeled actin. Biophys J. 1981 Aug;35(2):351–364. doi: 10.1016/S0006-3495(81)84794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHASHI K. MOLECULAR CHARACTERISTICS OF G-ADP ACTIN. Arch Biochem Biophys. 1964 Sep;107:441–448. doi: 10.1016/0003-9861(64)90300-5. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F. H. M. The molecular transformations of actin. III. The participation of nucleotides. J Biol Chem. 1952 Sep;198(1):469–475. [PubMed] [Google Scholar]
- Maruyama K. Effects of trace amounts of Ca2+ and Mg2+ on the polymerization of actin. Biochim Biophys Acta. 1981 Jan 30;667(1):139–142. doi: 10.1016/0005-2795(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Mihashi K., Wahl P. Nanosecond pulsefluorometry in polarized light of G-actin-epsilon-ATP and F-actin-epsilon-ADP. FEBS Lett. 1975 Mar 15;52(1):8–12. doi: 10.1016/0014-5793(75)80625-9. [DOI] [PubMed] [Google Scholar]
- Montague C., Rhee K. W., Carlson F. D. Measurement of the translational diffusion constant of G-actin by photon correlation spectroscopy. J Muscle Res Cell Motil. 1983 Feb;4(1):95–101. doi: 10.1007/BF00711960. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Huxley H. E., DeRosier D. J. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970 Jun 14;50(2):279–295. doi: 10.1016/0022-2836(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Oosawa F. Size distribution of protein polymers. J Theor Biol. 1970 Apr;27(1):69–86. doi: 10.1016/0022-5193(70)90129-3. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Mechanism of K+-induced actin assembly. J Cell Biol. 1982 Jun;93(3):648–654. doi: 10.1083/jcb.93.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- Rouayrenc J. F., Travers F. The first step in the polymerisation of actin. Eur J Biochem. 1981 May;116(1):73–77. doi: 10.1111/j.1432-1033.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tait J. F., Frieden C. Polymerization and gelation of actin studied by fluorescence photobleaching recovery. Biochemistry. 1982 Jul 20;21(15):3666–3674. doi: 10.1021/bi00258a022. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Reidler J., Spudich J. A., Stryer L. Detection of actin assembly by fluorescence energy transfer. J Cell Biol. 1981 May;89(2):362–367. doi: 10.1083/jcb.89.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Molecular cytochemistry: incorporation of fluorescently labeled actin into living cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):857–861. doi: 10.1073/pnas.75.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam R., Frieden C. Cytochalasin D and platelet gelsolin accelerate actin polymer formation. A model for regulation of the extent of actin polymer formation in vivo. Biochemistry. 1982 Jun 22;21(13):3207–3214. doi: 10.1021/bi00256a027. [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Lanni F., McNeil P. L., Ware B. R., Taylor D. L. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4660–4664. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Taylor D. L. Preparation and characterization of a new molecular cytochemical probe: 5-iodoacetamidofluorescein-labeled actin. J Histochem Cytochem. 1980 Nov;28(11):1198–1206. doi: 10.1177/28.11.6107318. [DOI] [PubMed] [Google Scholar]
- Wang Y. L., Taylor D. L. Probing the dynamic equilibrium of actin polymerization by fluorescence energy transfer. Cell. 1981 Dec;27(3 Pt 2):429–436. doi: 10.1016/0092-8674(81)90384-6. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wegner A., Savko P. Fragmentation of actin filaments. Biochemistry. 1982 Apr 13;21(8):1909–1913. doi: 10.1021/bi00537a032. [DOI] [PubMed] [Google Scholar]
- Wegner A. Spontaneous fragmentation of actin filaments in physiological conditions. Nature. 1982 Mar 18;296(5854):266–267. doi: 10.1038/296266a0. [DOI] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Hartwig J. H., Maruyama K., Stossel T. P. Ca2+ control of actin filament length. Effects of macrophage gelsolin on actin polymerization. J Biol Chem. 1981 Sep 25;256(18):9693–9697. [PubMed] [Google Scholar]


