Abstract
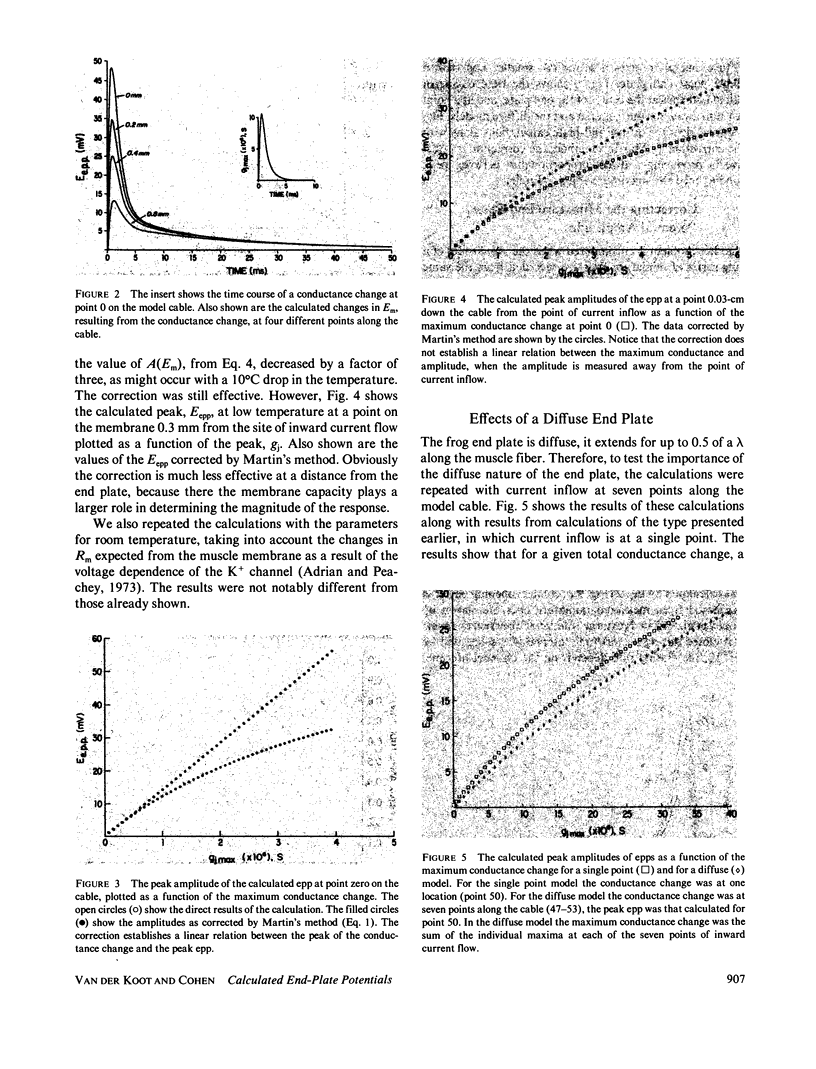
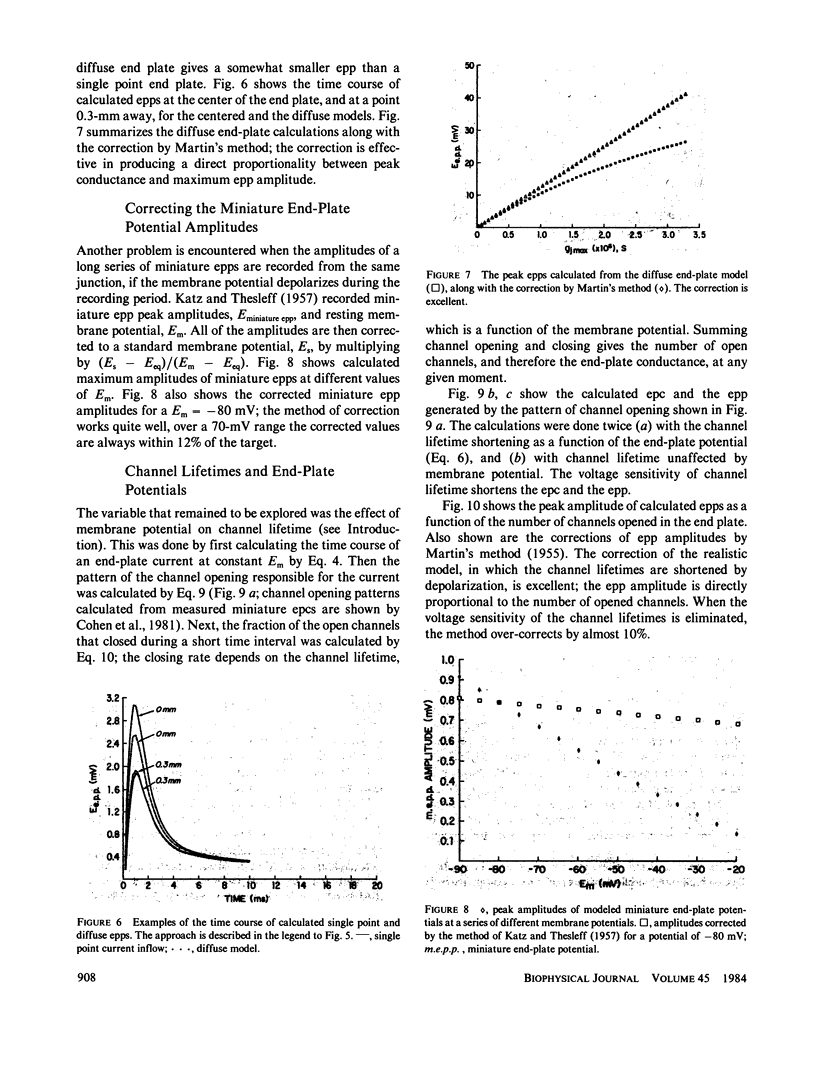
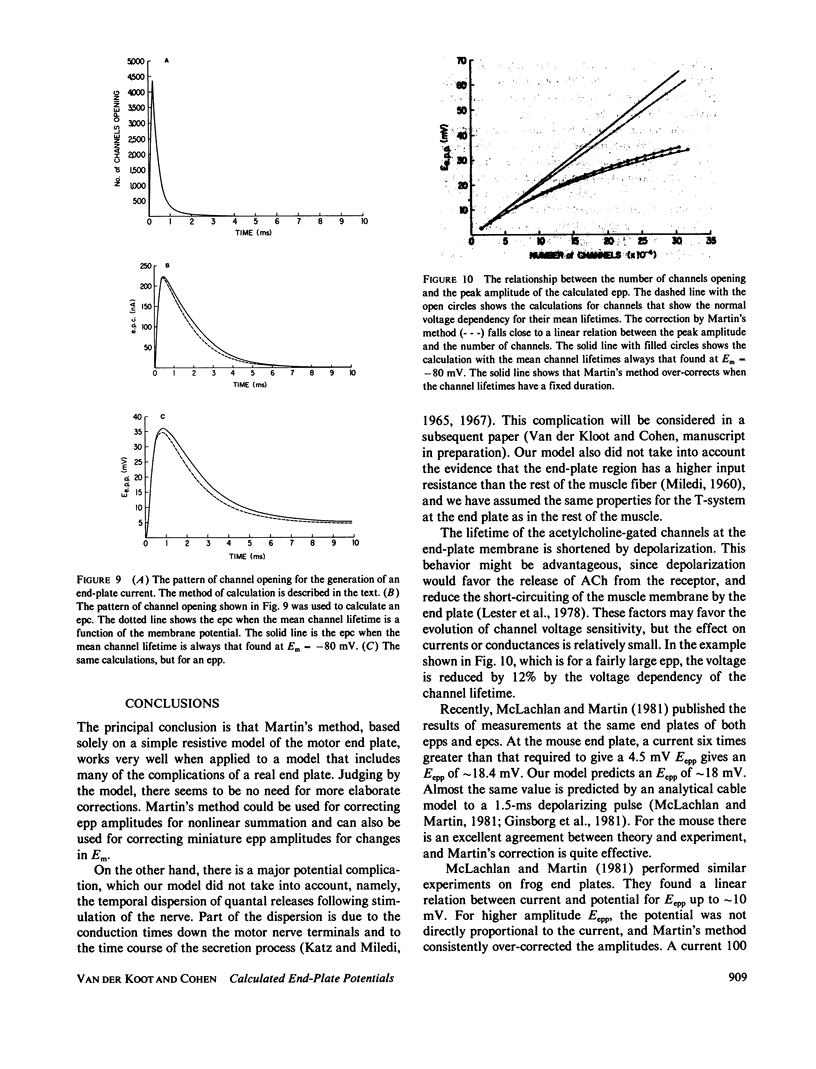
At the neuromuscular junction, the end-plate potential is generated by a conductance increase in the end-plate membrane. The end-plate depolarization brings the membrane potential toward the reversal potential, which diminishes the driving force for inward current flow. A. R. Martin (1955, J. Physiol. [Lond.]. 130:114-122) devised a simple formula to correct end-plate potential amplitudes for a diminished driving force based on a purely resistive model of the end-plate membrane. The model ignores the membrane capacity, the complexity of the equivalent circuit for a muscle fiber, the variation in channel lifetimes with changes in membrane potential, and the extension of the end plate along a length of the cable. We have developed a model that incorporates all of these features. The calculations show that Martin's correction is, in theory, quite satisfactory for a cable that has the characteristics of a muscle fiber unless the recording is made at a distance from the site of inward current flow. However, there is a discrepancy between models of the frog neuromuscular junction and the available experimental data, which suggests that the end-plate depolarization produced by a given current is greater than expected from their model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B. Upper and lower bounds on the non-linearity of summation of end-plate potentials. J Theor Biol. 1976 Nov;63(1):217–224. doi: 10.1016/0022-5193(76)90093-x. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Measurement of membrane capacity in skeletal muscle. Nat New Biol. 1973 Mar 14;242(115):62–64. doi: 10.1038/newbio242062a0. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., van der Kloot W., Attwell D. The timing of channel opening during miniature end-plate currents. Brain Res. 1981 Oct 26;223(1):185–189. doi: 10.1016/0006-8993(81)90821-0. [DOI] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., McLachlan E. M., Martin A. R., Searl J. W. The computation of simulated endplate potentials. Proc R Soc Lond B Biol Sci. 1981 Oct 14;213(1191):233–242. doi: 10.1098/rspb.1981.0064. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Koblin D. D., Sheridan R. E. Role of voltage-sensitive receptors in nicotinic transmission. Biophys J. 1978 Mar;21(3):181–194. doi: 10.1016/S0006-3495(78)85518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R. The effect of membrane capacitance on non-linear summation of synaptic potentials. J Theor Biol. 1976 Jun;59(1):179–187. doi: 10.1016/s0022-5193(76)80031-8. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Ramón F., Joyner R. W. Axon voltage-clamp simulations. I. Methods and tests. Biophys J. 1975 Jan;15(1):11–24. doi: 10.1016/S0006-3495(75)85788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. A comment on Martin's relation. Biophys J. 1976 Aug;16(8):891–895. doi: 10.1016/S0006-3495(76)85739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Bowen J. M. Effects of quantal unit latency on statistics of Poisson and binomial neurotransmitter release mechanisms. J Theor Biol. 1974 Jan;43(1):151–165. doi: 10.1016/s0022-5193(74)80050-0. [DOI] [PubMed] [Google Scholar]



