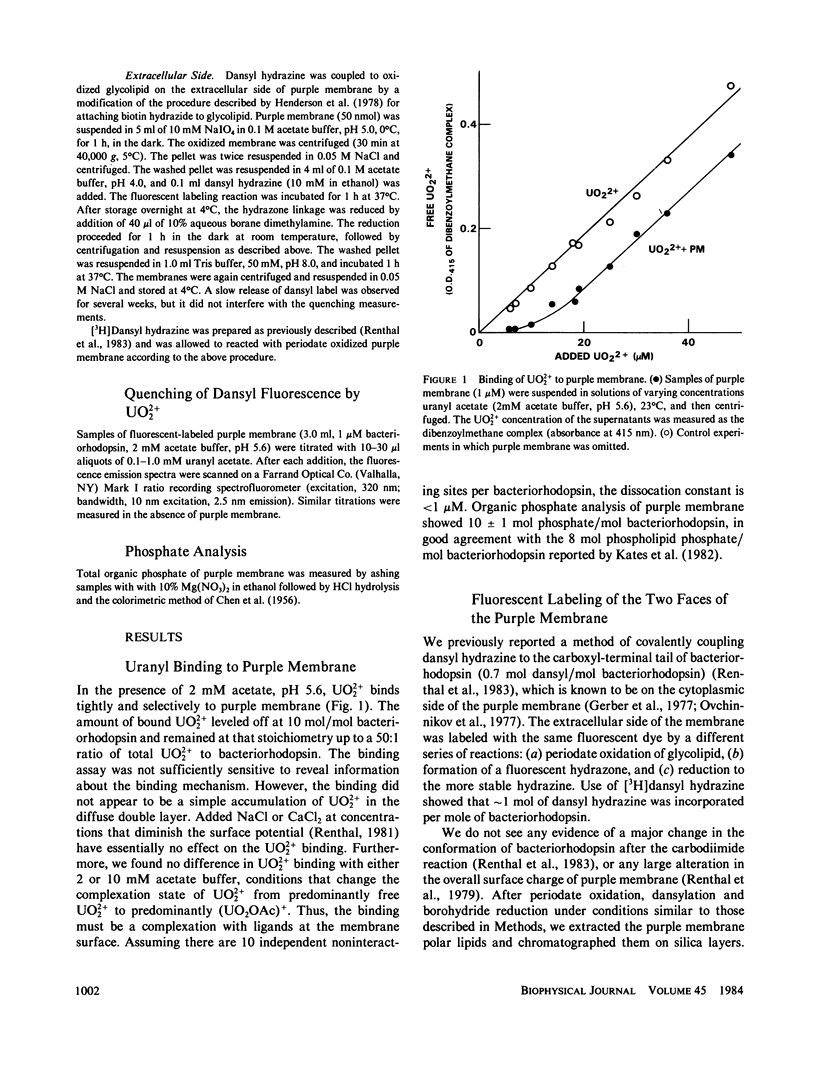
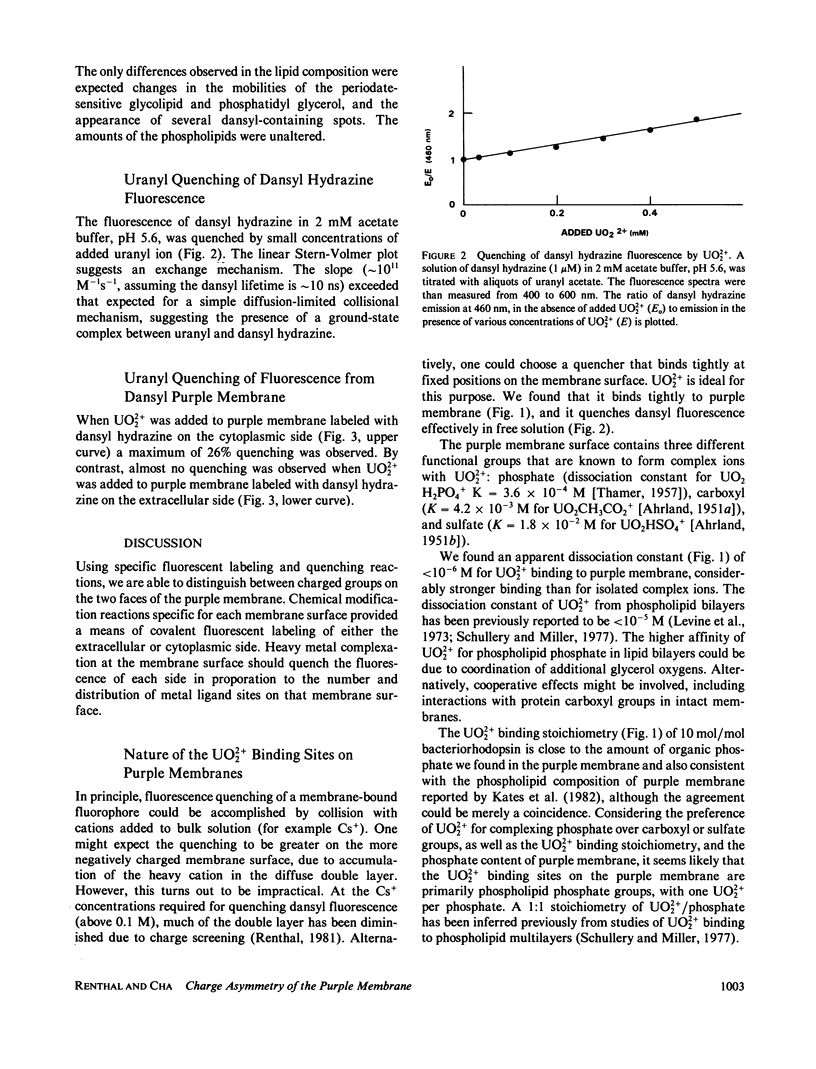
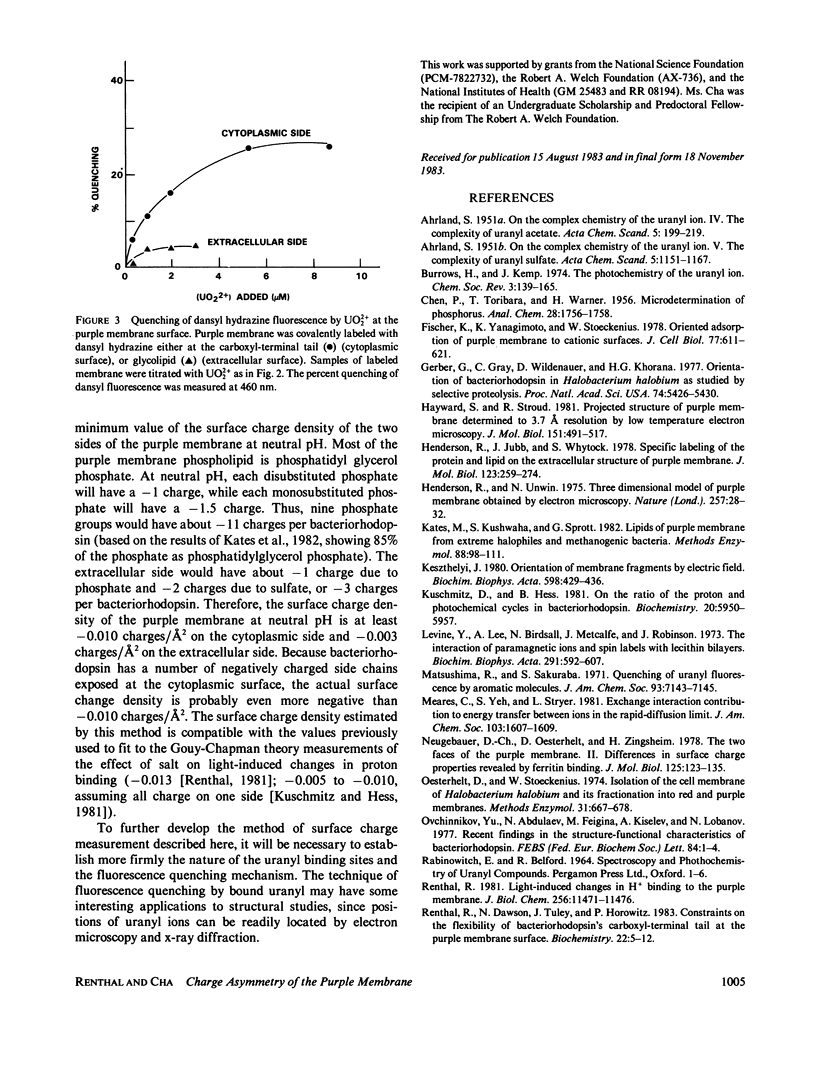
Abstract
Purple membrane was covalently labeled with 5-(dimethylamino) naphthalene-1-sulfonyl hydrazine (dansyl hydrazine) by carbodiimide coupling to the cytoplasmic surface (carboxyl-terminal tail: 0.7 mol/mol bacteriorhodopsin) or by periodate oxidation and dimethylaminoborane reduction at the extracellular surface (glycolipids: 1 mol/mol). In 2 mM acetate buffer, pH 5.6, micromolar concentrations of UO2 +(2) were found to quench the dansyl groups on the cytoplasmic surface (maximum = 26%), while little quenching was observed at the extracellular surface (maximum = 4%). Uranyl ion quenched dansyl hydrazine in free solution at much higher concentrations. Uranyl also bound tightly to unmodified purple membrane, (apparent dissociation constant = 0.8 microM) as measured by a centrifugation assay. The maximum stoichiometry was 10 mol/mol of bacteriorhodopsin, which is close to the amount of phospholipid phosphorus in purple membrane. The results were analyzed on the assumptions that UO2 +(2) binds in a 1:1 complex with phospholipid phosphate and that the dansyl distribution and quenching mechanisms are the same at both surfaces. This indicates a 9:1 ratio of phosphate between the cytoplasmic and extracellular surfaces. Thus, the surface change density of the cytoplasmic side of the membrane is more negative than -0.010 charges/A2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fisher K. A., Yanagimoto K., Stoeckenius W. Oriented adsorption of purple membrane to cationic surfaces. J Cell Biol. 1978 May;77(2):611–621. doi: 10.1083/jcb.77.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G. E., Gray C. P., Wildenauer D., Khorana H. G. Orientation of bacteriorhodopsin in Halobacterium halobium as studied by selective proteolysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5426–5430. doi: 10.1073/pnas.74.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Jubb J. S., Whytock S. Specific labelling of the protein and lipid on the extracellular surface of purple membrane. J Mol Biol. 1978 Aug 5;123(2):259–274. doi: 10.1016/0022-2836(78)90325-x. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Keszthelyi L. Orientation of membrane fragments by electric field. Biochim Biophys Acta. 1980 Jun 6;598(3):429–436. doi: 10.1016/0005-2736(80)90023-1. [DOI] [PubMed] [Google Scholar]
- Kuschmitz D., Hess B. On the ratio of the proton and photochemical cycles in bacteriorhodopsin. Biochemistry. 1981 Oct 13;20(21):5950–5957. doi: 10.1021/bi00524a005. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Lee A. G., Birdsall N. J., Metcalfe J. C., Robinson J. D. The interaction of paramagnetic ions and spin labels with lecithin bilayers. Biochim Biophys Acta. 1973 Feb 16;291(3):592–607. doi: 10.1016/0005-2736(73)90464-1. [DOI] [PubMed] [Google Scholar]
- Neugebauer D. C., Oesterhelt D., Zingsheim H. P. The two faces of the purple membrane. II. Differences in surface charge properties revealed by ferritin binding. J Mol Biol. 1978 Oct 25;125(2):123–135. doi: 10.1016/0022-2836(78)90341-8. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Renthal R., Dawson N., Tuley J., Horowitz P. Constraints on the flexibility of bacteriorhodopsin's carboxyl-terminal tail at the purple membrane surface. Biochemistry. 1983 Jan 4;22(1):5–12. doi: 10.1021/bi00270a601. [DOI] [PubMed] [Google Scholar]
- Renthal R., Harris G. J., Parrish R. Reaction of the purple membrane with a carbodiimide. Biochim Biophys Acta. 1979 Aug 14;547(2):258–269. doi: 10.1016/0005-2728(79)90009-4. [DOI] [PubMed] [Google Scholar]
- Renthal R. Light-induced changes in H+ binding to the purple membrane. Effect of pH, light, temperature, and ionic strength. J Biol Chem. 1981 Nov 25;256(22):11471–11476. [PubMed] [Google Scholar]
- Schullery S. E., Miller R. H. Binding of uranyl to phosphatidylcholine liposomes. Liposome aggregation effect on surface area. Biochim Biophys Acta. 1977 Aug 1;468(3):451–460. doi: 10.1016/0005-2736(77)90294-2. [DOI] [PubMed] [Google Scholar]
- Shinar R., Druckmann S., Ottolenghi M., Korenstein R. Electric field effects in bacteriorhodopsin. Biophys J. 1977 Jul;19(1):1–5. doi: 10.1016/S0006-3495(77)85558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]


