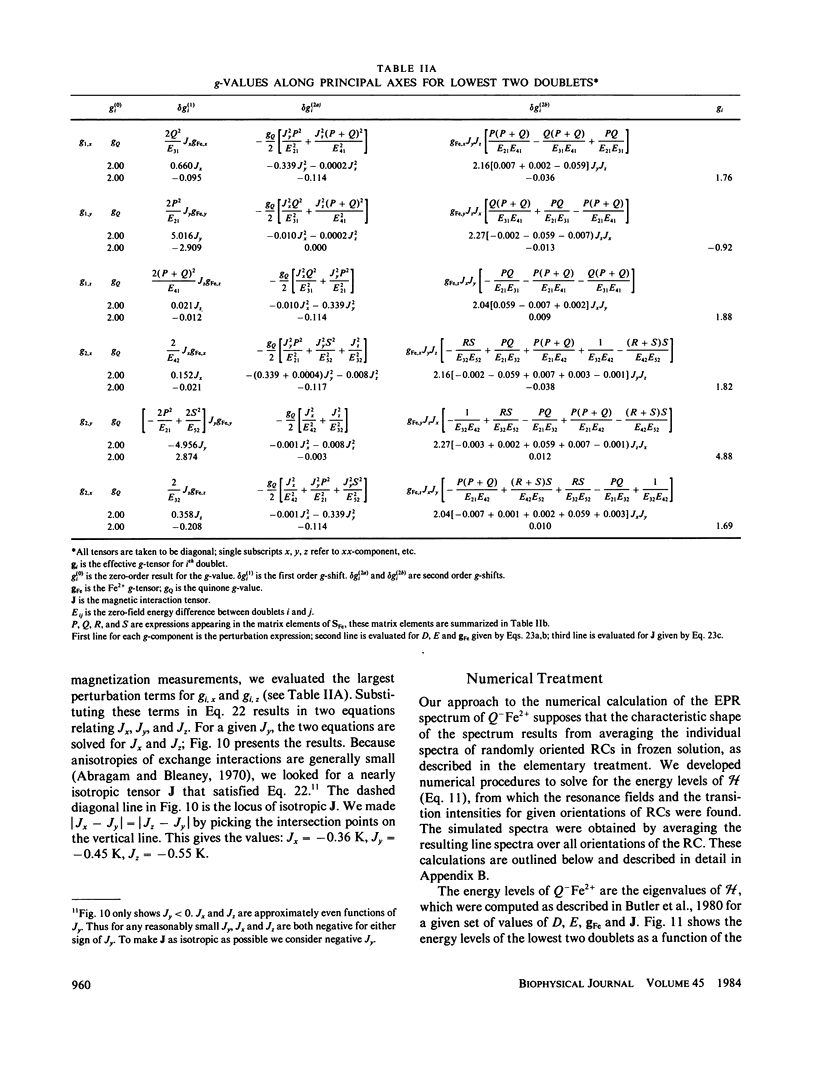
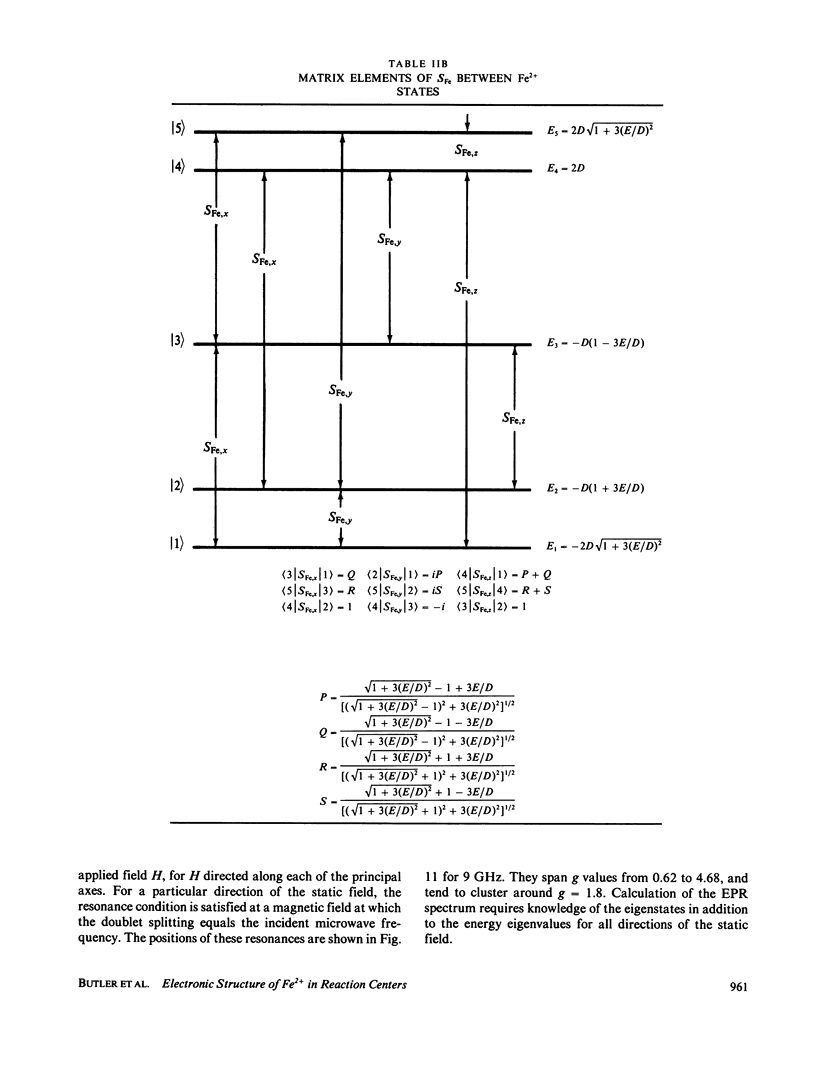
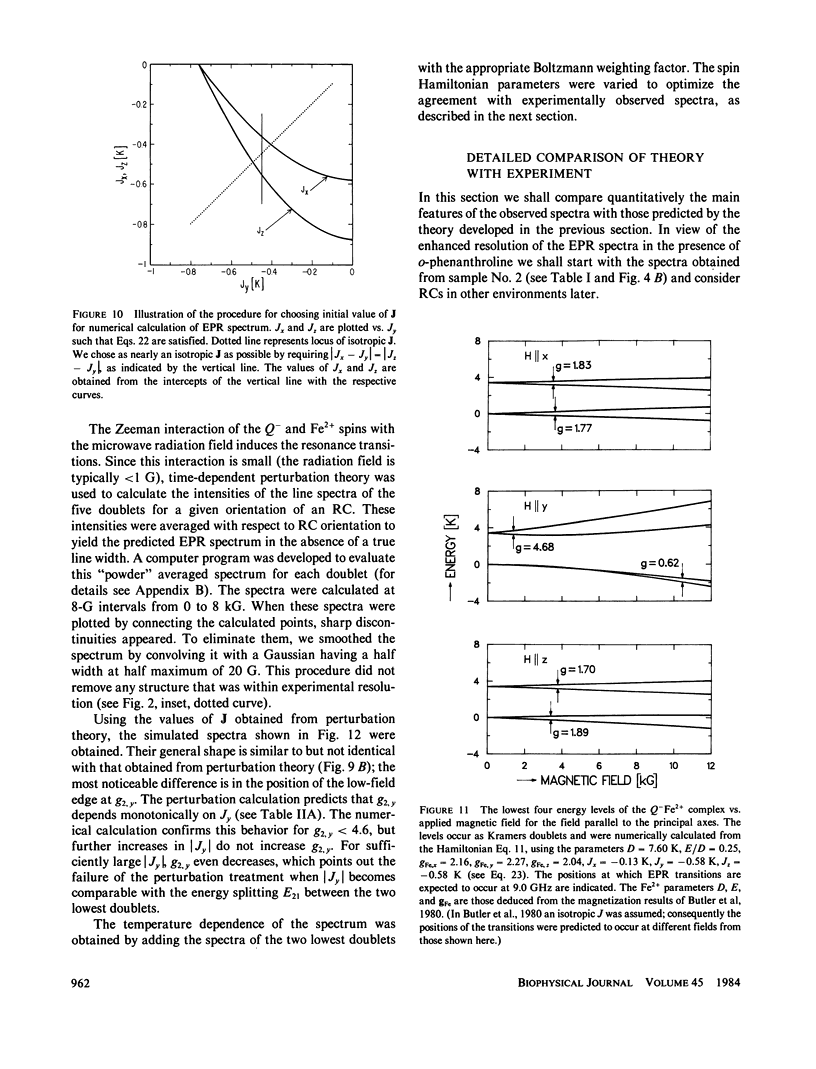
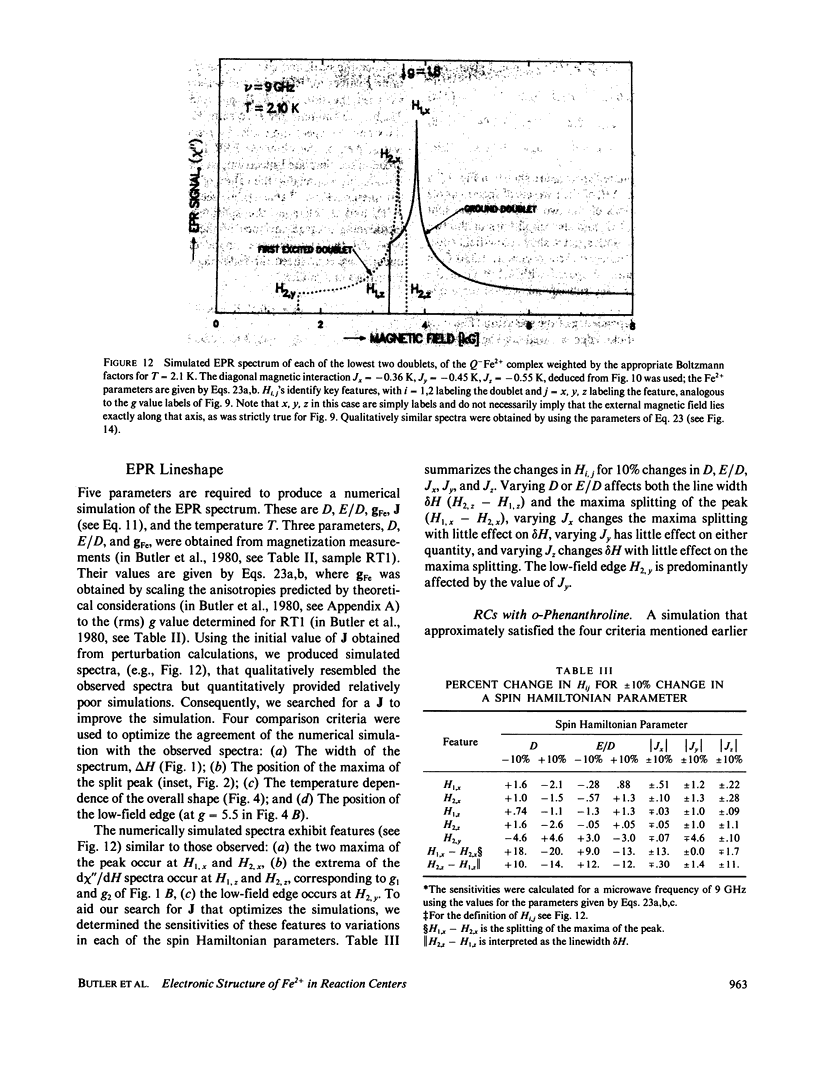
Abstract
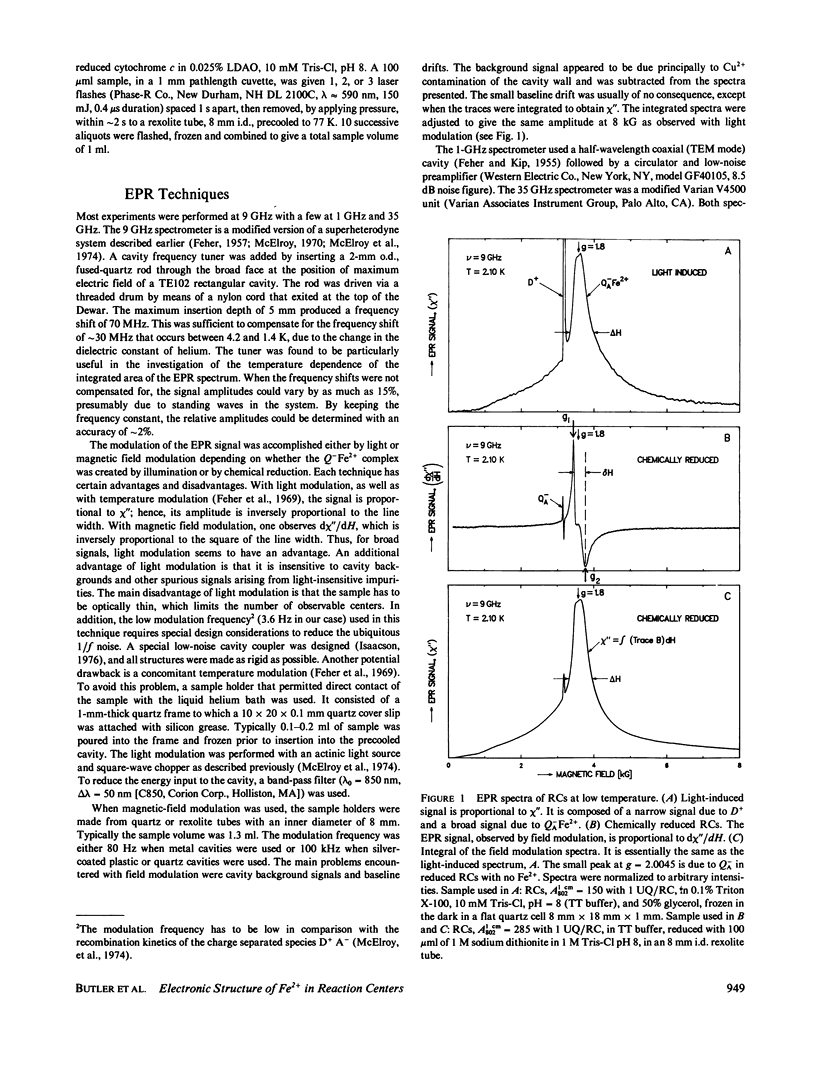
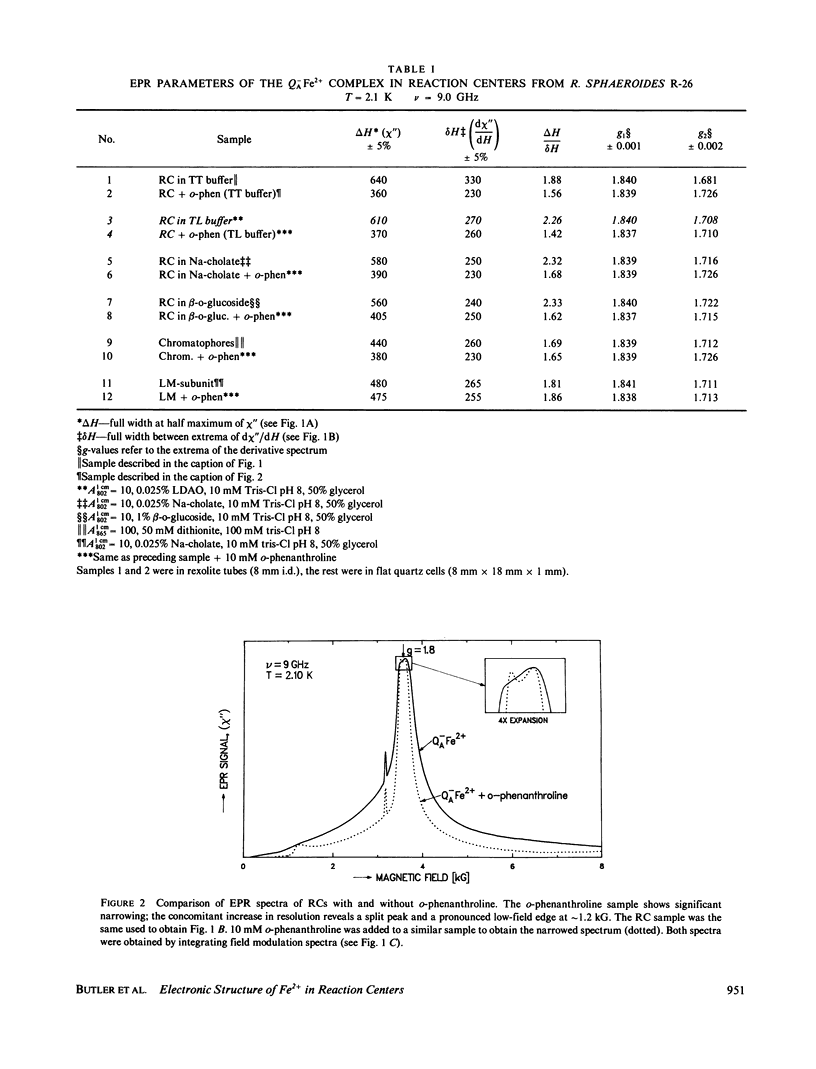
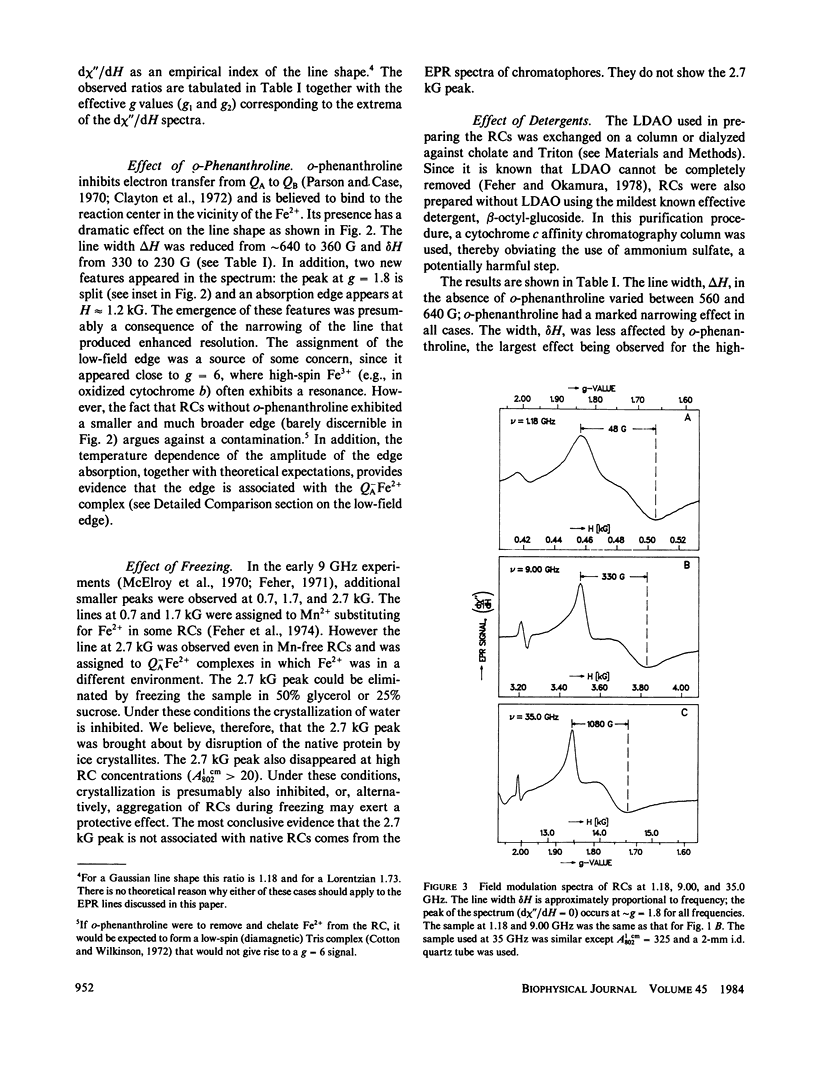
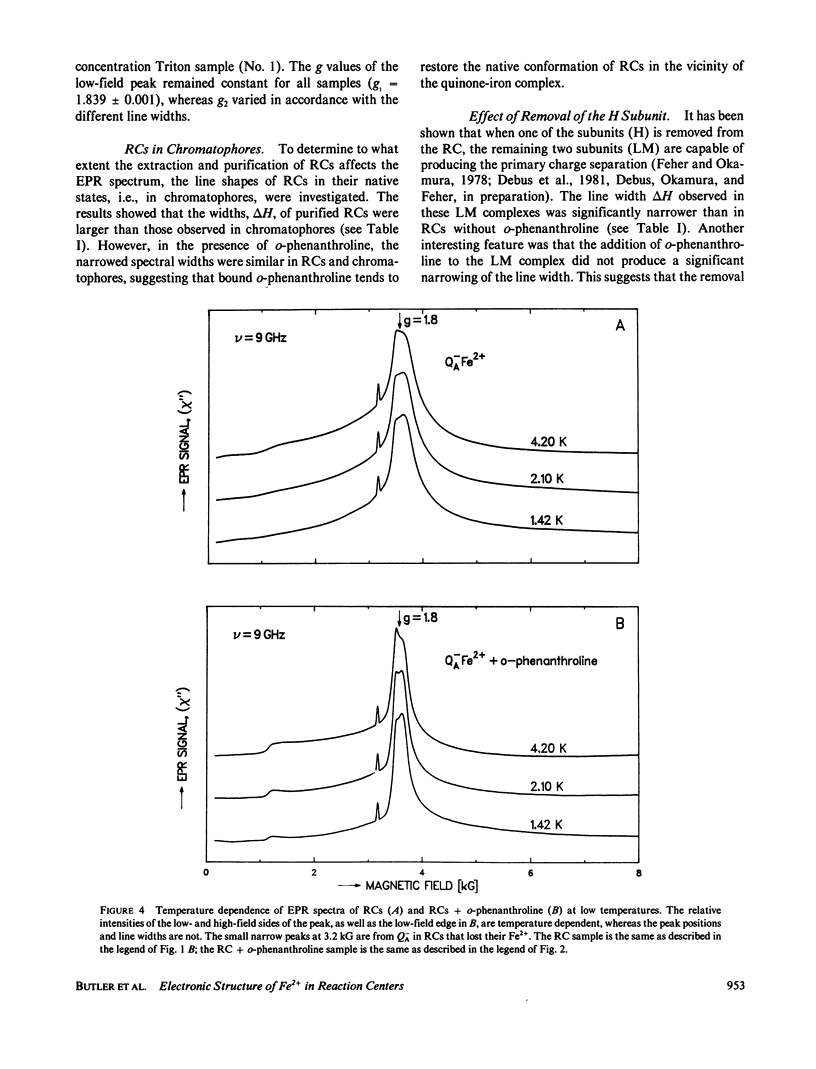
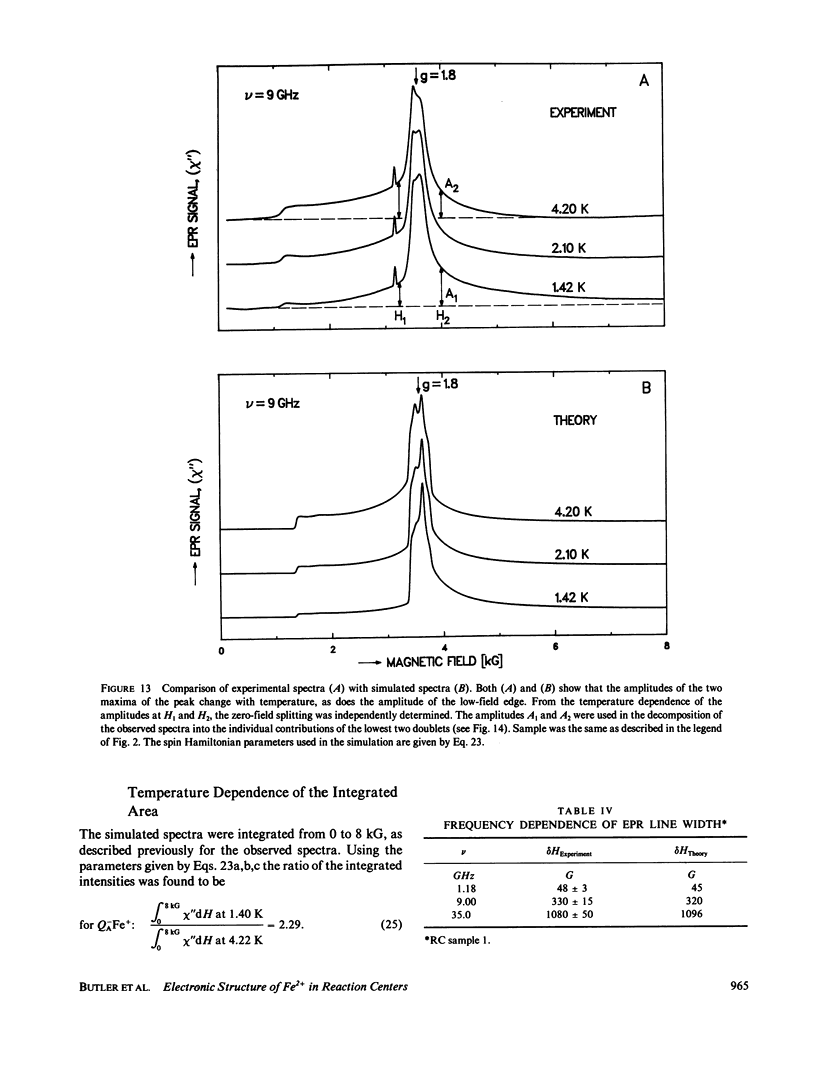
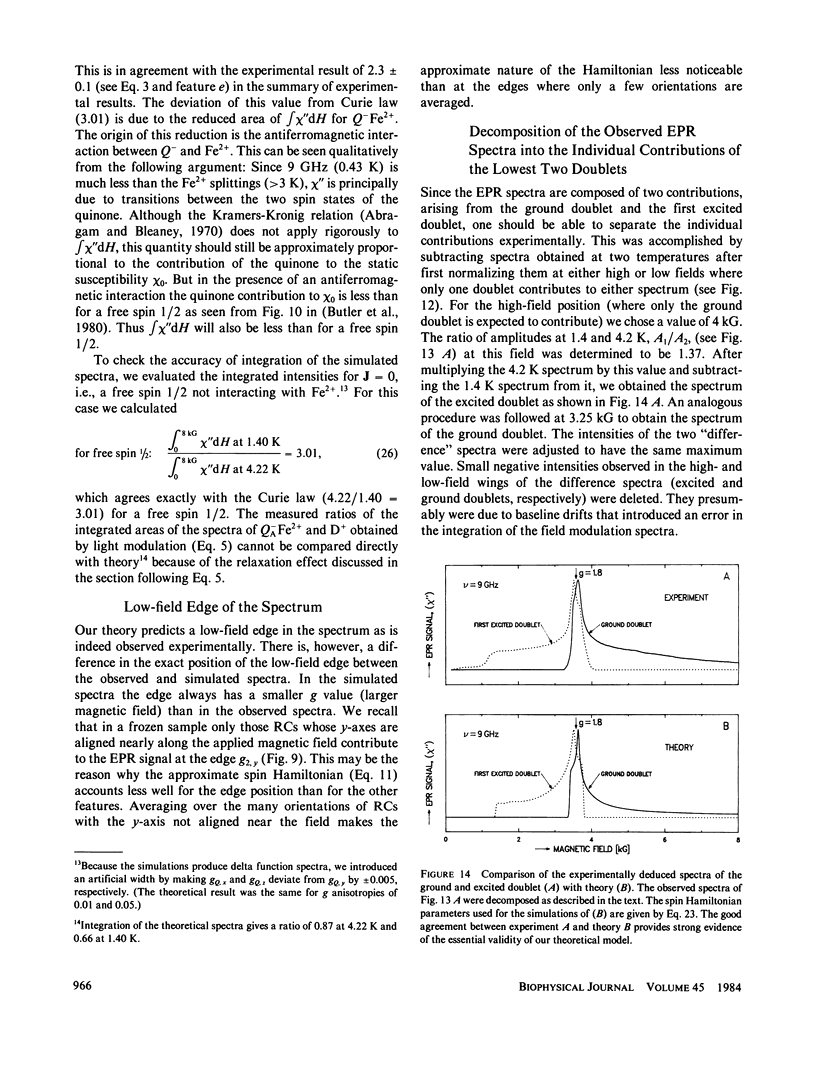
Electron paramagnetic resonance (EPR) spectra of the reduced quinone-iron acceptor complex in reaction centers were measured in a variety of environments and compared with spectra calculated from a theoretical model. Spectra were obtained at microwave frequencies of 1, 9, and 35 GHz and at temperatures from 1.4 to 30 K. The spectra are characterized by a broad absorption peak centered at g = 1.8 with wings extending from g approximately equal to 5 to g less than 0.8. The peak is split with the low-field component increasing in amplitude with temperature. The theoretical model is based on a spin Hamiltonian, in which the reduced quinone, Q-, interacts magnetically with Fe2+. In this model the ground manifold of the interacting Q-Fe2+ system has two lowest doublets that are separated by approximately 3 K. Both perturbation analyses and exact numerical calculations were used to show how the observed spectrum arises from these two doublets. The following spin Hamiltonian parameters optimized the agreement between simulated and observed spectra: the electronic g tensor gFe, x = 2.16, gFe, y = 2.27, gFez = 2.04, the crystal field parameters D = 7.60 K and E/D = 0.25, and the antiferromagnetic magnetic interaction tensor, Jx = -0.13 K, Jy = -0.58 K, Jz = -0.58 K. The model accounts well for the g value (1.8) of the broad peak, the observed splitting of the peak, the high and low g value wings, and the observed temperature dependence of the shape of the spectra. The structural implications of the value of the magnetic interaction, J, and the influence of the environment on the spin Hamiltonian parameters are discussed. The similarity of spectra and relaxation times observed from the primary and secondary acceptor complexes Q-AFe2+ and Fe2+Q-B leads to the conclusion that the Fe2+ is approximately equidistant from QA and QB.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brudvig G. W., Worland S. T., Sauer K. Procedure for rapid isolation of photosynthetic reaction centers using cytochrome c affinity chromatography. Proc Natl Acad Sci U S A. 1983 Feb;80(3):683–686. doi: 10.1073/pnas.80.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker G., Stern E. A., Blankenship R. E., Parson W. W. An x-ray absorption study of the iron site in bacterial photosynthetic reaction centers. Biophys J. 1982 Feb;37(2):539–551. doi: 10.1016/S0006-3495(82)84699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. F., Johnston D. C., Shore H. B., Fredkin D. R., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. I. Static magnetization measurements. Biophys J. 1980 Dec;32(3):967–992. doi: 10.1016/S0006-3495(80)85030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Straley S. C. Photochemical electron transport in photosynthetic reaction centers. IV. Observations related to the reduced photoproducts. Biophys J. 1972 Oct;12(10):1221–1234. doi: 10.1016/S0006-3495(72)86158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Szuts E. Z., Fleming H. Photochemical electron transport oin photosynthetic reaction centers from Rhodopseudomonas spheroides. 3. Effects of orthophenanthroline and other chemicals. Biophys J. 1972 Jan;12(1):64–79. doi: 10.1016/s0006-3495(72)86071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Brune D. C., Clayton R. K. Effects of extraction and replacement of ubiquinone upon the photochemical activity of reaction centers and chromatophores from Rhodopseudomonas spheriodes. FEBS Lett. 1974 Sep 1;45(1):344–347. doi: 10.1016/0014-5793(74)80877-x. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Jr, Reed D. W. Primary events in the photosynthetic reaction centre from Rhodopseudomonas spheroides strain R26: triplet and oxidized states of bacteriochlorophyll and the identification of the primary electron acceptor. Biochim Biophys Acta. 1973 Apr 5;292(3):654–664. doi: 10.1016/0005-2728(73)90013-3. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. II. Extended x-ray fine structure studies. Biophys J. 1982 Feb;37(2):523–538. doi: 10.1016/S0006-3495(82)84698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher G., Hoff A. J., Isaacson R. A., Ackerson L. C. ENDOR experiments on chlorophyll and bacteriochlorophyll in vitro and in the photosynthetic unit. Ann N Y Acad Sci. 1975 Apr 15;244:239–259. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]
- Feher G., Isaacson R. A., McElroy J. D., Ackerson L. C., Okamura M. Y. On the question of the primary acceptor in bacterial photosynthesis:manganese substituting for iron in reaction centers of Rhodopseudomonas spheroides R-26. Biochim Biophys Acta. 1974 Oct 18;368(1):135–139. doi: 10.1016/0005-2728(74)90104-2. [DOI] [PubMed] [Google Scholar]
- Feher G., Okamura M. Y., McElroy J. D. Identification of an electron acceptor in reaction centers of Rhodopseudomonas spheroides by EPR spectroscopy. Biochim Biophys Acta. 1972 Apr 20;267(1):222–226. doi: 10.1016/0005-2728(72)90155-7. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Halsey Y. D., Parson W. W. Identification of ubiquinone as the secondary electron acceptor in the photosynthetic apparatus of Chromatium vinosum. Biochim Biophys Acta. 1974 Jun 28;347(3):404–416. doi: 10.1016/0005-2728(74)90079-6. [DOI] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Dutton P. L. The primary electron acceptor in photosynthesis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):414–421. doi: 10.1016/s0006-291x(72)80154-2. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Hall R. L. The question of the primary electron acceptor in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):786–790. doi: 10.1073/pnas.69.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. Characterization of primary reactants in bacterial photosynthesis. I. Comparison of the light-induced EPR signal (g=2.0026) with that of a bacteriochlorophyll radical. Biochim Biophys Acta. 1972 May 25;267(2):363–374. doi: 10.1016/0005-2728(72)90123-5. [DOI] [PubMed] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. doi: 10.1016/0022-2836(82)90216-9. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Druyan M. E., Katz J. J. Electron nuclear double resonance of bacteriochlorophyll free radical in vitro and in vivo. J Am Chem Soc. 1973 Mar 7;95(5):1680–1682. doi: 10.1021/ja00786a066. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Katz J. J. Models for antenna and reaction center chlorophylls. Ann N Y Acad Sci. 1975 Apr 15;244:260–280. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Thurnauer M. C., Bowman M. K. Electron spin echo spectroscopy and the study of biological structure and function. Adv Biol Med Phys. 1980;17:365–416. doi: 10.1016/b978-0-12-005217-2.50015-4. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Case G. D. In Chromatium, a single photochemical reaction center oxidizes both cytochrome C552 and cytochrome C555. Biochim Biophys Acta. 1970;205(2):232–245. doi: 10.1016/0005-2728(70)90253-7. [DOI] [PubMed] [Google Scholar]
- Rutherford A. W., Evans M. C. Direct measurement of the redox potential of the primary and secondary quinone electron acceptors in Rhodopseudomonas sphaeroides (wild-type) by EPR spectrometry. FEBS Lett. 1980 Feb 11;110(2):257–261. doi: 10.1016/0014-5793(80)80086-x. [DOI] [PubMed] [Google Scholar]
- Slooten L. Electron acceptors in reaction center preparations from photosynthetic bacteria. Biochim Biophys Acta. 1972 Aug 17;275(2):208–218. doi: 10.1016/0005-2728(72)90042-4. [DOI] [PubMed] [Google Scholar]
- Vermeglio A. Secondary electron transfer in reaction centers of Rhodopseudomonas sphaeroides. Out-of-phase periodicity of two for the formation of ubisemiquinone and fully reduced ubiquinone. Biochim Biophys Acta. 1977 Mar 11;459(3):516–524. doi: 10.1016/0005-2728(77)90050-0. [DOI] [PubMed] [Google Scholar]
- Wraight C. A. Electron acceptors of photosynthetic bacterial reaction centers. Direct observation of oscillatory behaviour suggesting two closely equivalent ubiquinones. Biochim Biophys Acta. 1977 Mar 11;459(3):525–531. doi: 10.1016/0005-2728(77)90051-2. [DOI] [PubMed] [Google Scholar]


