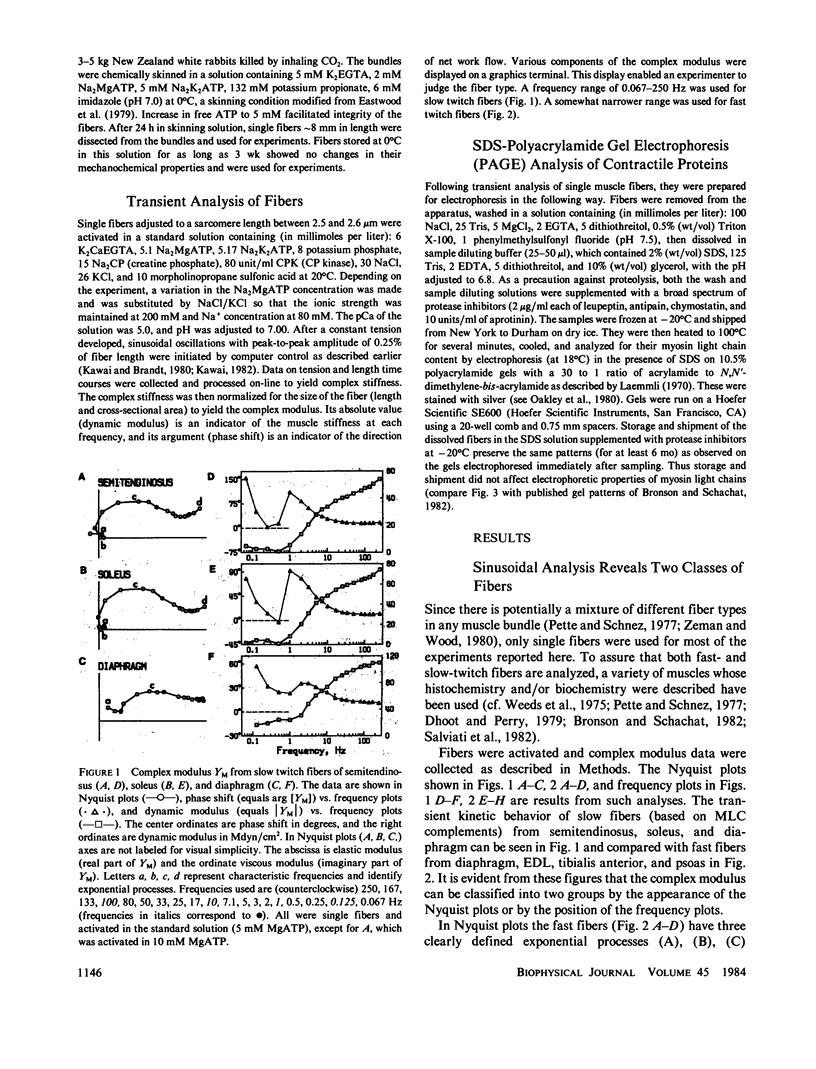
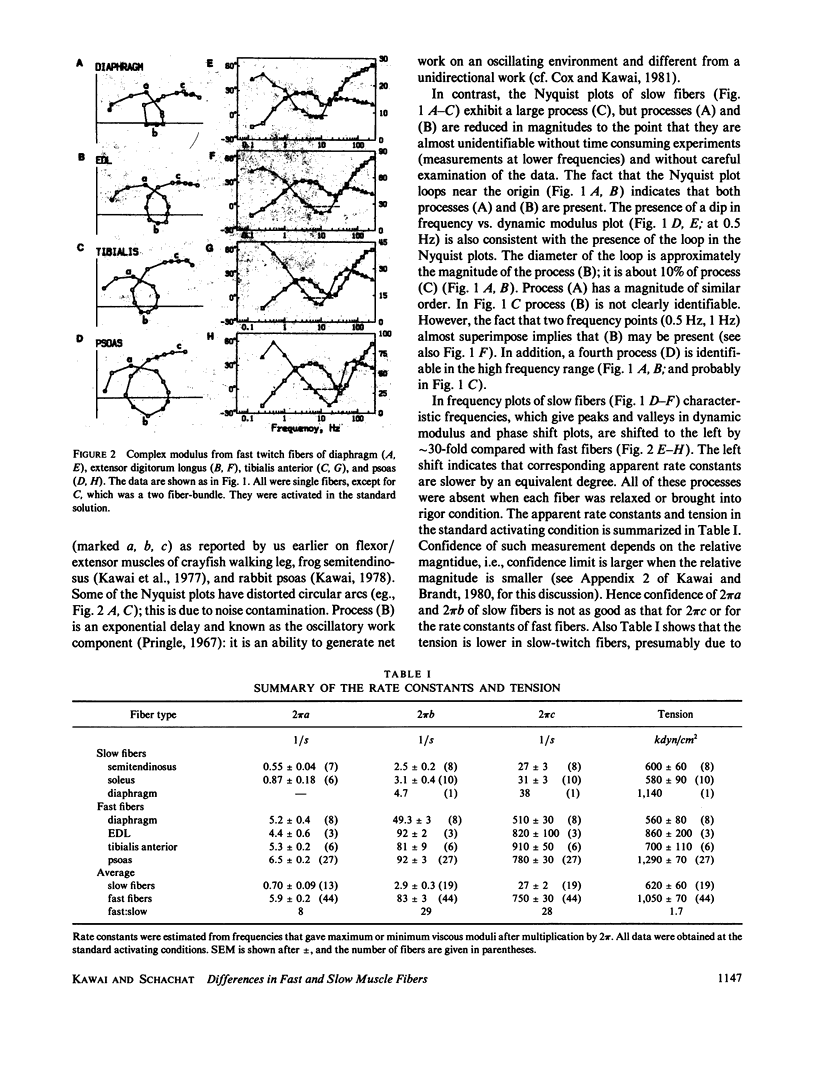
Abstract
Sinusoidal analysis of the mechanochemical properties of skinned muscle fibers under conditions of maximal activation was applied to fibers from several rabbit skeletal muscles (psoas, tibialis anterior, extensor digitorum longus, diaphragm, soleus, semitendinosus). This investigation distinguished between two general classes of fibers, which on the basis of their myosin light chain complements could be classified as fast and slow. In fast fibers (e.g., psoas) we identified the presence of at least three exponential processes (A), (B), (C) of comparable magnitudes. In slow fibers (e.g., soleus) we identified the presence of at least four exponential processes (A)-(D) of very different magnitudes; magnitudes of processes (A) and (B) are very small compared with those of (C) and (D). The apparent rate constants are 8-29-fold slower in slow fibers. Because our sinusoidal characterization takes less than or equal to 22 s and does not involve chemical denaturation or other means of disruption of the myofilament lattice, it allows the different physiological classes of fibers to be characterized and then studied further by other techniques. The perfect correlation between physiological and molecular properties as assayed by gel electrophoresis after sinusoidal analysis demonstrates this and justifies its use in distinguishing between fiber types.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronson D. D., Schachat F. H. Heterogeneity of contractile proteins. Differences in tropomyosin in fast, mixed, and slow skeletal muscles of the rabbit. J Biol Chem. 1982 Apr 10;257(7):3937–3944. [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway J. E., Bechtel P. J. C-protein from rabbit soleus (red) muscle. Biochem J. 1981 May 1;195(2):463–469. doi: 10.1042/bj1950463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cox R. N., Kawai M. Alternate energy transduction routes in chemically skinned rabbit psoas muscle fibres: a further study of the effect of MgATP over a wide concentration range. J Muscle Res Cell Motil. 1981 Jun;2(2):203–214. doi: 10.1007/BF00711870. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature. 1979 Apr 19;278(5706):714–718. doi: 10.1038/278714a0. [DOI] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl P., Kuhn H. J., Rüegg J. C. Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol. 1974 Mar;237(2):243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Waller G. S. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol. 1981 Feb;311:201–218. doi: 10.1113/jphysiol.1981.sp013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980 Sep;1(3):279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawai M., Brandt P., Orentlicher M. Dependence of energy transduction in intact skeletal muscles on the time in tension. Biophys J. 1977 May;18(2):161–172. doi: 10.1016/S0006-3495(77)85605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M. Head rotation or dissociation? A study of exponential rate processes in chemically skinned rabbit muscle fibers when MgATP concentration is changed. Biophys J. 1978 Apr;22(1):97–103. doi: 10.1016/S0006-3495(78)85473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Secrist D., Coby R., Lucas S. Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult. Nature. 1976 Apr 1;260(5550):440–441. doi: 10.1038/260440a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol. 1980 Jun 5;139(4):573–600. doi: 10.1016/0022-2836(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pette D., Schnez U. Myosin light chain patterns of individual fast and slow-twitch fibres of rabbit muscles. Histochemistry. 1977 Oct 22;54(2):97–107. doi: 10.1007/BF00489668. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibres. An electrophoretic study of single fibres. Biochem J. 1982 Nov 1;207(2):261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
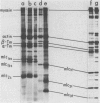
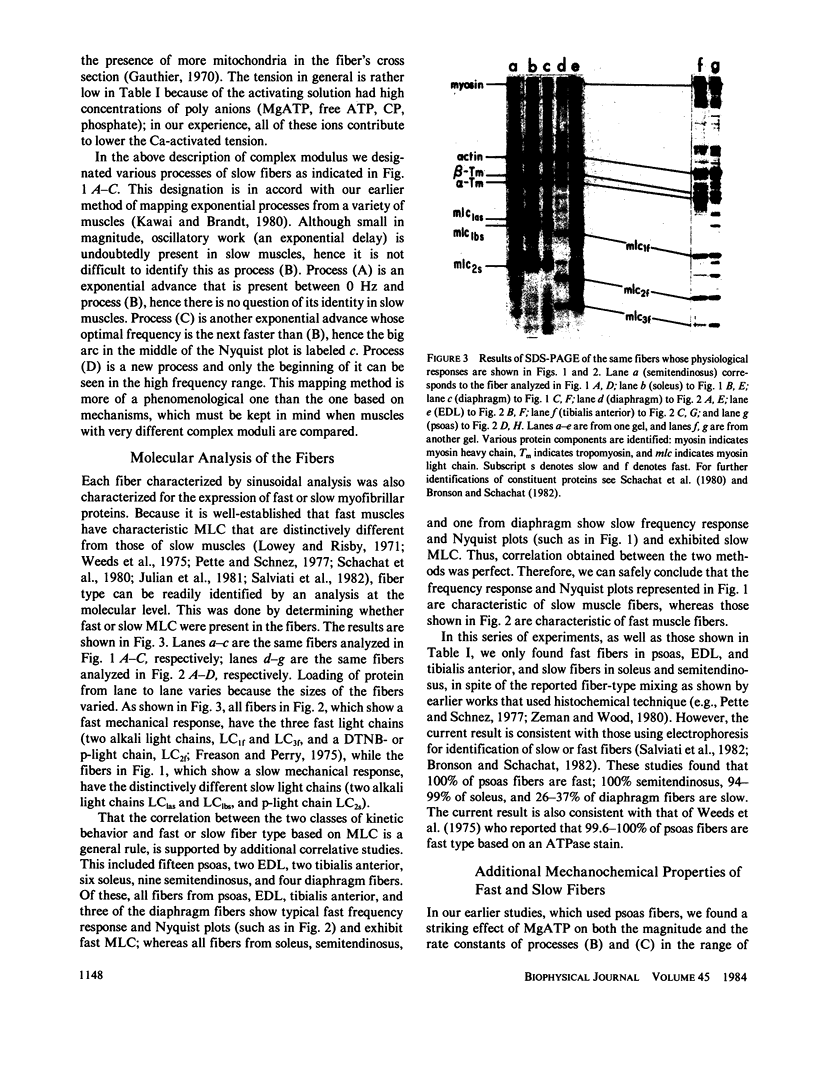
- Schachat F. H., Bronson D. D., McDonald O. B. Two kinds of slow skeletal muscle fibers which differ in their myosin light chain complements. FEBS Lett. 1980 Dec 15;122(1):80–82. doi: 10.1016/0014-5793(80)80406-6. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hall R., Spurway N. C. Characterization of myosin light chains from histochemically identified fibres of rabbit psoas muscle. FEBS Lett. 1975 Jan 1;49(3):320–324. doi: 10.1016/0014-5793(75)80776-9. [DOI] [PubMed] [Google Scholar]
- Zeman R. J., Wood D. S. Correlative histochemical and physiological measurements in single skinned muscle fibers: Heterogeneity of Ca sensitivity in type I fibers. J Histochem Cytochem. 1980 Jul;28(7):714–715. doi: 10.1177/28.7.6446576. [DOI] [PubMed] [Google Scholar]